Abstract
Purpose
To compare the clinical benefit and tolerability of triple therapy of lenvatinib, programmed death 1 (PD-1) inhibitor, and transarterial chemoembolization (TACE) versus dual therapy of lenvatinib and PD-1 inhibitor in unresectable hepatocellular carcinoma (HCC) patients.
Methods
Between October 2018 and September 2021, patients with unresectable HCC who received triple therapy of lenvatinib, PD-1 inhibitor, and TACE or dual therapy of lenvatinib and PD-1 inhibitor participated in this study. The efficacy was evaluated by survival and therapeutic response, and the tolerability was evaluated by the frequency and severity of key adverse events (AEs).
Results
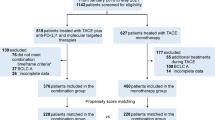
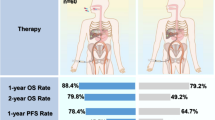
In total, 118 eligible patients with unresectable HCC who received combination therapy were included in this study. Among them, 60 patients received triple therapy of lenvatinib, PD-1 inhibitor, and TACE (L–P–T group), and 58 eligible patients received dual therapy of lenvatinib and PD-1 inhibitor (L–P group). Patients who received triple therapy had better overall survival (OS) [median, 29.0 vs. 17.8 months, p < 0.01] and progression-free survival (PFS) [median, 16.2 vs. 10.2 months, p < 0.01] than those who received dual therapy. The objective response rate (76.7 vs. 44.9%, p < 0.01) and disease control rate (96.7 vs. 75.9%, p < 0.01) in the L–P–T group were higher than in the L–P group, respectively. Multivariate analyses revealed that the treatment option and BCLC stage were independent prognostic factors for OS, while treatment option and tumor number were independent prognostic factors for PFS. The incidence and severity of AEs in the L–P–T group were comparable to those in the L–P group (any grade, 95.0 vs. 94.8%, p = 1.00; grade ≥ 3, 30.0 vs. 27.6%, p = 0.93).
Conclusion
Triple therapy of lenvatinib, PD-1 inhibitor, and TACE may achieve more favorable survival benefits than dual therapy of lenvatinib and PD-1 inhibitor in unresectable HCC patients with manageable safety profiles.




Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 2021;71(3):209–249.
Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019;380(15):1450–1462.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology (Baltimore, MD) 2005;42(5):1208–1236.
EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol 2018;69(1):182–236.
Chung GE, Lee J-H, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 2011;258(2):627–634.
Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69(8):1492–1501.
Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hep Intl 2021;15(3):663–675.
Kudo M, Han K-H, Ye S-L, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer 2020;9(3):245–260.
Zhang S, Zhao Y, He L, et al. Effect of camrelizumab plus transarterial chemoembolization on massive hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2022;46(4): 101851.
Ke Q, Xin F, Fang H, Zeng Y, Wang L, Liu J. The significance of transarterial chemo(embolization) combined with tyrosine kinase inhibitors and immune checkpoint inhibitors for unresectable hepatocellular carcinoma in the era of systemic therapy: a systematic review. Front Immunol 2022;13: 913464.
Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15(10):599–616.
Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 2022;19(3):151–172.
Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compreh Cancer Netw 2021;19(5):541–565.
Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18(8):525–543.
Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19(7):940–952.
Yau T, Park J-W, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022;23(1):77–90.
Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59(1):81–88.
Yau T, Kang Y-K, Kim T-Y, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol 2020;6(11): e204564.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382(20):1894–1905.
Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol 2021;22(7):977–990.
Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol 2020;38(26):2960–2970.
Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res 2021;27(4):1003–1011.
Huinen ZR, Huijbers EJM, van Beijnum JR, Nowak-Sliwinska P, Griffioen AW. Anti-angiogenic agents—overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol 2021;18(8):527–540.
Wu C-J, Lee P-C, Hung Y-W, et al. Lenvatinib plus pembrolizumab for systemic therapy-naïve and -experienced unresectable hepatocellular carcinoma. Cancer Immunol Immunother 2022;71(11):2631–2643.
Cheng A-L, Hsu C, Chan SL, Choo S-P, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol 2020;72(2):307–319.
Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18(5):293–313.
Tischfield DJ, Gurevich A, Johnson O, et al. Transarterial embolization modulates the immune response within target and nontarget hepatocellular carcinomas in a rat model. Radiology 2022;303(1):215–225.
Cai M, Huang W, Huang J, et al. Transarterial Chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol 2022;13: 848387.
Xiang Y-J, Wang K, Yu H-M, et al. Transarterial chemoembolization plus a PD-1 inhibitor with or without lenvatinib for intermediate-stage hepatocellular carcinoma. Hepatol Res 2022;52(8):721–729.
Sun L, Xu X, Meng F, et al. Lenvatinib plus transarterial chemoembolization with or without immune checkpoint inhibitors for unresectable hepatocellular carcinoma: a review. Front Oncol 2022;12: 980214.
Li X, Fu Z, Chen X, et al. Efficacy and safety of lenvatinib combined with PD-1 inhibitors plus TACE for unresectable hepatocellular carcinoma patients in China real-world. Front Oncol 2022;12: 950266.
Teng Y, Ding X, Li W, Sun W, Chen J. A retrospective study on therapeutic efficacy of transarterial chemoembolization combined with immune checkpoint inhibitors plus lenvatinib in patients with unresectable hepatocellular carcinoma. Technol Cancer Res Treat 2022;21:15330338221075174.
Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, MD) 2018;67(1):358–380.
Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol 2020;72(2):288–306.
Angeli P, et al. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69(2):406–460.
Matsuki M, Hoshi T, Yamamoto Y, et al. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med 2018;7(6):2641–2653.
Wang Y, Jiang M, Zhu J, et al. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed Pharmacother 2020;132:110797.
Xia Y, Tang W, Qian X, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer 2022;10(4):e004656.
Shimose S, Iwamoto H, Tanaka M, et al. Alternating lenvatinib and trans-arterial therapy prolongs overall survival in patients with inter-mediate stage hepatocellular carcinoma: a propensity score matching study. Cancers 2021;13(1):160.
Jácome AA, Castro ACG, Vasconcelos JPS, et al. Efficacy and safety associated with immune checkpoint inhibitors in unresectable hepatocellular carcinoma: a meta-analysis. JAMA Netw Open 2021;4(12): e2136128.
Chen J, Hu X, Li Q, et al. Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients. Annals Transl Med 2020;8(18):1187.
Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer 2022;21(1):28.
Funding
This project is funded by Key Technology Research and Development Program of Shandong, ZR2022QF114.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yujing Xin, Xinyuan Zhang, Ning Liu,Gang Peng, Xiaoyu Huang, Xiaojing Cao, Xiang Zhou and Xiao Li declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethical approval
The ethics committee approved the ethics of this retrospective study, and all participants signed informed consent for treatment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xin, Y., Zhang, X., Liu, N. et al. Efficacy and safety of lenvatinib plus PD-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma. Hepatol Int 17, 753–764 (2023). https://doi.org/10.1007/s12072-023-10502-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10502-3