Abstract
Background
Extracellular vesicles (EVs) play pivotal roles in tumor growth, cancer metastasis and angiogenesis. Here, we aimed to identify proteins that contribute to the functionality of EVs derived from metastatic hepatocellular carcinoma (HCC) cells.
Methods
Proteins of EVs derived from metastatic HCC cells and normal liver cells were analyzed by mass spectrometry. Proteomic profiling identified actin-related protein 2/3 complex subunit 2 (ARPC2) to be highly expressed in EVs of metastatic HCC cells. The expression of ARPC2 in EVs and HCC tissues was examined using immunoblotting and TCGA database, respectively. The functional roles of EV-ARPC2 were investigated by knockout approach and various in vitro and in vivo assays.
Results
ARPC2 was highly expressed in EVs of metastatic cells but barely detected in non-metastatic HCC cells and normal liver cells. Immunogold labeling showed the presence of APRC2 on the surface of EVs. Analysis of TCGA database of liver cancer revealed ARPC2 overexpression was correlated with poor prognosis of patients. ARPC2 was knockout in metastatic HCC cells. EVs derived from knockout cells displayed compromised activity in enhancing cell growth, motility and metastasis compared to EVs of control cells. Pimozide, an inhibitor of APRC2, also inhibited the promoting effect of EVs of metastatic cells in lung colonization of tumor cells in mice.
Conclusion
This study reveals previously unreported expression and function of ARPC2 in EVs. EVs with highly expressed ARPC2 enhance cancer cell growth and metastasis. ARPC2 may provide a prospective target for the novel treatment of HCC patients.
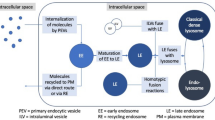
Graphical abstract






Similar content being viewed by others
Availability of data and material
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease Study. JAMA Oncol. 2017;3(4):524–548
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424
Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29(1):62–67
Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. 2020;371:m3544
Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2020;17(3):139–152
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228
Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182(4):1044-1061.e1018
Lee YT, Tran BV, Wang JJ, Liang IY, You S, Zhu Y, et al. The role of extracellular vesicles in disease progression and detection of hepatocellular carcinoma. Cancers (Basel). 2021;13(12):3076
Tian XP, Wang CY, Jin XH, Li M, Wang FW, Huang WJ, et al. Acidic microenvironment up-regulates exosomal miR-21 and mir-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics. 2019;9(7):1965–1979
Sugimachi K, Matsumura T, Hirata H, Uchi R, Ueda M, Ueo H, et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer. 2015;112(3):532–538
Huang XY, Huang ZL, Huang J, Xu B, Huang XY, Xu YH, et al. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39(1):20
Guo Q, Furuta K, Lucien F, Gutierrez Sanchez LH, Hirsova P, Krishnan A, et al. Integrin β(1)-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol. 2019;71(6):1193–1205
Mao X, Tey SK, Yeung CLS, Kwong EML, Fung YME, Chung CYS, et al. Nidogen 1-enriched extracellular vesicles facilitate extrahepatic metastasis of liver cancer by activating pulmonary fibroblasts to secrete tumor necrosis factor receptor 1. Adv Sci (Weinh). 2020;7(21):2002157
Mao X, Zhou L, Tey SK, Ma APY, Yeung CLS, Ng TH, et al. Tumour extracellular vesicle-derived Complement Factor H promotes tumorigenesis and metastasis by inhibiting complement-dependent cytotoxicity of tumour cells. J Extracell Vesicles. 2020;10(1):e12031
Tey SK, Wong SWK, Chan JYT, Mao X, Tung HN, Yeung CLS, et al. Patient pIgR-enriched extracellular vesicles drive cancer stemness, tumorigenesis and metastasis in hepatocellular carcinoma. J Hepatol. 2022;76(4):883–895
Robinson RC, Turbedsky K, Kaiser DA, Marchand JB, Higgs HN, Choe S, et al. Crystal structure of Arp2/3 complex. Science. 2001;294(5547):1679–1684
Huang S, Li D, Zhuang L, Sun L, Wu J. Identification of Arp2/3 complex subunits as prognostic biomarkers for hepatocellular carcinoma. Front Mol Biosci. 2021;8:690151
Cancer Genome Atlas Research N, Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45(10):1113–1120
Lian Q, Wang S, Zhang G, Wang D, Luo G, Tang J, et al. HCCDB: a database of hepatocellular carcinoma expression atlas. Genom Proteom Bioinform. 2018;16(4):269–275
Suraneni P, Rubinstein B, Unruh JR, Durnin M, Hanein D, Li R. The Arp2/3 complex is required for lamellipodia extension and directional fibroblast cell migration. J Cell Biol. 2012;197(2):239–251
Frank DJ, Hopmann R, Lenartowska M, Miller KG. Capping protein and the Arp2/3 complex regulate nonbundle actin filament assembly to indirectly control actin bundle positioning during Drosophila melanogaster bristle development. Mol Biol Cell. 2006;17(9):3930–3939
Liu Z, Yang X, Chen C, Liu B, Ren B, Wang L, et al. Expression of the Arp2/3 complex in human gliomas and its role in the migration and invasion of glioma cells. Oncol Rep. 2013;30(5):2127–2136
Chen P, Yue X, Xiong H, Lu X, Ji Z. RBM3 upregulates ARPC2 by binding the 3’UTR and contributes to breast cancer progression. Int J Oncol. 2019;54(4):1387–1397
Helgeson LA, Nolen BJ. Mechanism of synergistic activation of Arp2/3 complex by cortactin and N-WASP. Elife. 2013;2:e00884
Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M, et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol. 2016;214(2):197–213
Cheng Z, Wei W, Wu Z, Wang J, Ding X, Sheng Y, et al. ARPC2 promotes breast cancer proliferation and metastasis. Oncol Rep. 2019;41(6):3189–3200
Zhang J, Liu Y, Yu CJ, Dai F, Xiong J, Li HJ, et al. Role of ARPC2 in human gastric cancer. Mediat Inflamm. 2017;2017:5432818
Choi J, Lee YJ, Yoon YJ, Kim CH, Park SJ, Kim SY, et al. Pimozide suppresses cancer cell migration and tumor metastasis through binding to ARPC2, a subunit of the Arp2/3 complex. Cancer Sci. 2019;110(12):3788–3801
Yoon YJ, Han YM, Choi J, Lee YJ, Yun J, Lee SK, et al. Benproperine, an ARPC2 inhibitor, suppresses cancer cell migration and tumor metastasis. Biochem Pharmacol. 2019;163:46–59
Acknowledgements
The authors thank The University of Hong Kong, Li Ka Shing Faculty of Medicine, Centre for PanorOmic Sciences Imaging and Flow Cytometry Core for providing facility for animal imaging. The authors also thank Centre for Comparative Medicine Research for providing facility for animal experiments and the Electron Microscope Unit for providing service and support needed for experiments involving electron microscope.
Funding
The work was supported by National Natural Science Foundation of China (NSFC) General Program (Grant number: 81872340 and 82072626).
Author information
Authors and Affiliations
Contributions
PM, SKT, XM and JWPY designed the study. PM, SWKW, THN, CLSY, YX, LY, QH and PC conducted investigation and collected data. PM drafted the manuscript. JWPY, SKT and YG contributed to the critical revision of the manuscript. JWPY obtained funding. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Piaorong Mei, Sze Keong Tey, Samuel Wan Ki Wong, Tung Him Ng, Xiaowen Mao, Cherlie Lot Sum Yeung, Yi Xu, Liang Yu, Qianhua Huang, Peihua Cao, Judy Wai Ping Yam, Yi Gao have nothing to disclose.
Ethics approval
All animal studies were approved by the Committee of the Use of Live Animals in Teaching and Research (CULATR), The University of Hong Kong. All animal work and procedures were followed strictly according to the Animals (Control of Experiments) Ordinance (Hong Kong) and the Institute’s guidance from Centre for Comparative Medical Research (CCMR), Li Ka Shing Faculty of Medicine, The University of Hong Kong.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mei, P., Tey, S.K., Wong, S.W.K. et al. Actin-related protein 2/3 complex subunit 2-enriched extracellular vesicles drive liver cancer metastasis. Hepatol Int 16, 603–613 (2022). https://doi.org/10.1007/s12072-022-10338-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10338-3