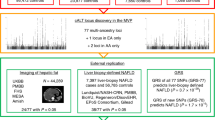
Abstract
Background
A genetic variant in the manganese transporter SLC30A10 (rs188273166, p.Thr95Ile) was associated with increased plasma alanine transaminase (ALT) in a recent genome-wide association study in the UK Biobank (UKB). The aims of the present study were to test the association of rs188273166 with ALT in an independent cohort, and to begin to assess the clinical, hepatic, and biochemical phenotypes associated with the variant.
Methods
We included n = 334,886 white participants from UKB, including 14,462 with hepatic magnetic resonance imaging (MRI), and n = 113,612 individuals from the Copenhagen City Heart Study and the Copenhagen General Population Study combined.
Results
Genotyping SLC30A10 p.Thr95Ile identified 816 heterozygotes in the UKB and 111 heterozygotes in the Copenhagen cohort. Compared to noncarriers, heterozygotes had 4 and 5 U/L higher levels of ALT in the UKB and Copenhagen cohort, respectively, and 3 U/L higher plasma aspartate transaminase and gamma-glutamyl transferase in the UKB. Heterozygotes also had higher corrected T1 on liver MRI, a marker of hepatic inflammation (p = 4 × 10–7), but no change in MRI-quantified steatosis (p = 0.57). Plasma manganese was within the normal range in nine heterozygotes that provided new blood samples. SLC30A10 p.Thr95Ile heterozygotes had an eightfold increased risk of biliary tract cancer in UKB (p = 4 × 10–7), but this association was not replicated in the Copenhagen cohort.
Conclusions
SLC30A10 p.Thr95Ile was associated with elevated liver enzymes in two large general population cohorts, and with MRI-quantified hepatic inflammation.
Graphical abstract
A rare genetic variant (p.Thr95Ile) in the manganese transporter SLC30A10 is associated with elevated plasma alanine transaminase (ALT) and higher corrected T1 on liver MRI, markers of liver inflammation. These data support that the variant may increase the risk of liver disease.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- CCHS:
-
Copenhagen City Heart Study
- CGPS:
-
Copenhagen General Population Study
- PDFF:
-
Proton density fat fraction
- PNPLA3:
-
Patatin-like phospholipase domain-containing protein 3
- SLC30A10:
-
Solute Carrier Family 30 Member 10
- UKB:
-
UK Biobank
References
Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136(5):1585–1592
Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, et al. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology. 2015;149(7):1784–1793
Jamialahmadi O, Mancina RM, Ciociola E, Tavaglione F, Luukkonen PK, Baselli G, et al. Exome-wide association study on alanine aminotransferase identifies sequence variants in the GPAM and APOE associated with fatty liver disease. Gastroenterology. 2021;160:1634–1646
Parisinos CA, Wilman HR, Thomas EL, Kelly M, Nicholls RC, McGonigle J, et al. Genome-wide and Mendelian randomisation studies of liver MRI yield insights into the pathogenesis of steatohepatitis. J Hepatol. 2020;73(2):241–251
Emdin CA, Haas M, Ajmera V, Simon TG, Homburger J, Neben C, et al. Association of genetic variation with cirrhosis: a multi-trait genome-wide association and gene-environment interaction study. Gastroenterology. 2020;160:1620-1633.e13
Anstee QM, Darlay R, Cockell S, Meroni M, Govaere O, Tiniakos D, et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J Hepatol. 2020;73(3):505–515
Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378(12):1096–1106
Buch S, Stickel F, Trepo E, Way M, Herrmann A, Nischalke HD, et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47(12):1443–1448
Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46(4):352–356
Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465
Sinnott-Armstrong N, Tanigawa Y, Amar D, Mars N, Benner C, Aguirre M, et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat Genet. 2021;53(2):185–194
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209
Stender S, Kozlitina J, Nordestgaard BG, Tybjaerg-Hansen A, Hobbs HH, Cohen JC. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat Genet. 2017;49(6):842–847
Gellert-Kristensen H, Richardson TG, Davey Smith G, Nordestgaard BG, Tybjaerg-Hansen A, Stender S. Combined effect of PNPLA3, TM6SF2, and HSD17B13 variants on risk of cirrhosis and hepatocellular carcinoma in the general population. Hepatology. 2020;72:845–856
Wechphanich S, Thammarat P. A survey of metal contamination in blood collection tubes on toxicology assays. BKK Med J. 2017. https://doi.org/10.31524/bkkmedj.2017.09.002
Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 4th ed. Elsevier Saunders, New York; 2006.
Wilman HR, Parisinos CA, Atabaki-Pasdar N, Kelly M, Thomas EL, Neubauer S, et al. Genetic studies of abdominal MRI data identify genes regulating hepcidin as major determinants of liver iron concentration. J Hepatol. 2019;71(3):594–602
Mojtahed A, Kelly CJ, Herlihy AH, Kin S, Wilman HR, McKay A, et al. Reference range of liver corrected T1 values in a population at low risk for fatty liver disease—a UK Biobank sub-study, with an appendix of interesting cases. Abdom Radiol (NY). 2019;44(1):72–84
Wilman HR, Kelly M, Garratt S, Matthews PM, Milanesi M, Herlihy A, et al. Characterisation of liver fat in the UK Biobank cohort. PLoS ONE. 2017;12(2):e0172921
Seidelin AS, Nordestgaard BG, Tybjaerg-Hansen A, Stender S. Genetic variation at PPP1R3B increases hepatic CT attenuation and interacts with prandial status on plasma glucose. J Clin Endocrinol Metab. 2020;105(6):dgaa151
Fuchs A, Mejdahl MR, Kuhl JT, Stisen ZR, Nilsson EJ, Kober LV, et al. Normal values of left ventricular mass and cardiac chamber volumes assessed by 320-detector computed tomography angiography in the Copenhagen general population study. Eur Heart J Cardiovasc Imaging. 2016;17(9):1009–1017
Ruhl CE, Everhart JE. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatology. 2012;55(2):447–454
Lawrence EM, Pooler BD, Pickhardt PJ. Opportunistic screening for hereditary hemochromatosis with unenhanced CT: determination of an optimal liver attenuation threshold. AJR Am J Roentgenol. 2018;211(6):1206–1211
Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, et al. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet. 2012;90(3):467–477
Tuschl K, Clayton PT, Gospe SM Jr, Gulab S, Ibrahim S, Singhi P, et al. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet. 2012;90(3):457–466
Ward LD, Tu HC, Quenneville CB, Tsour S, Flynn-Carroll AO, Parker MM, et al. GWAS of serum ALT and AST reveals an association of SLC30A10 Thr95Ile with hypermanganesemia symptoms. Nat Commun. 2021;12(1):4571
Tavasoli A, Arjmandi Rafsanjani K, Hemmati S, Mojbafan M, Zarei E, Hosseini S. A case of dystonia with polycythemia and hypermanganesemia caused by SLC30A10 mutation: a treatable inborn error of manganese metabolism. BMC Pediatr. 2019;19(1):229
Anagianni S, Tuschl K. Genetic disorders of manganese metabolism. Curr Neurol Neurosci Rep. 2019;19(6):33
Mercadante CJ, Prajapati M, Conboy HL, Dash ME, Herrera C, Pettiglio MA, et al. Manganese transporter Slc30a10 controls physiological manganese excretion and toxicity. J Clin Investig. 2019;129(12):5442–5461
Hutchens S, Liu C, Jursa T, Shawlot W, Chaffee BK, Yin W, et al. Deficiency in the manganese efflux transporter SLC30A10 induces severe hypothyroidism in mice. J Biol Chem. 2017;292(23):9760–9773
Pilling LC, Tamosauskaite J, Jones G, Wood AR, Jones L, Kuo CL, et al. Common conditions associated with hereditary haemochromatosis genetic variants: cohort study in UK Biobank. BMJ. 2019;364:k5222
Skowronska M, Litwin T, Kurkowska-Jastrzebska I, Czlonkowska A. Transcranial sonography changes in heterozygotic carriers of the ATP7B gene. Neurol Sci. 2020;41(9):2605–2612
Lambrianides S, Nicolaou P, Michaelidou M, Kakouris P, Votsi C, Petrou PP, et al. A novel SLC30A10 missense variant associated with parkinsonism and dystonia without hypermanganesemia. J Neurol Sci. 2020;418:117101
Ng E, Lind PM, Lindgren C, Ingelsson E, Mahajan A, Morris A, et al. Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum Mol Genet. 2015;24(16):4739–4745
Acknowledgements
We thank the staff and participants of the UKB, CGPS, and CCHS. This research has been conducted using the UK Biobank Resource (application identifiers 9914 and 15825). We thank Per Bo Jensen for help with the ICP-MS analyses of manganese.
Funding
This work was supported by Independent Research Fund Denmark and the Research Fund at Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark. Stefan Stender is supported by a Sapere Aude Research Leader grant from Independent Research Fund Denmark (9060-00012B). Hanieh Yaghootkar is funded by Diabetes UK RD Lawrence fellowship (grant: 17/0005594). The funding organizations had no role in any of the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
AS: data curation, formal analysis, investigation, visualization, writing—original draft, writing—review and editing. BGN: resources, writing—review and editing. ATH: resources, writing—review and editing. HY: resources, formal analysis, writing—review and editing. SS: conceptualization, data curation, formal analysis, investigation, visualization, funding acquisition, supervision, writing—original draft, writing—review and editing. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Anne-Sofie Seidelin, Børge Grønne Nordestgaard, Anne Tybjærg-Hansen, Hanieh Yaghootkar and Stefan Stender have no relevant financial or non-financial interests to disclose.
Animal research
Not applicable.
Consent to participate
All participants in the Copenhagen cohort and UK Biobank provided written consent.
Consent to publish
All co-authors agreed to the final version of the manuscript, and to the decision to submit for publication.
Plant reproducibility
Not applicable.
Clinical trials registration
No applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seidelin, AS., Nordestgaard, B.G., Tybjærg-Hansen, A. et al. A rare genetic variant in the manganese transporter SLC30A10 and elevated liver enzymes in the general population. Hepatol Int 16, 702–711 (2022). https://doi.org/10.1007/s12072-022-10331-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10331-w