Abstract
Backgrounds
ALT ≥ 80 U/L and HBV DNA ≥ 2000 IU/ml are treatment criteria of APASL guidelines for chronic hepatitis B (CHB) patients. The need of antiviral therapy for patients in gray zone (ALT < 80 U/L or HBV DNA < 2000 IU/ml) is controversial. This study aimed to develop a scoring system to predict hepatocellular carcinoma (HCC) and evaluate the benefit of antiviral therapy in these patients.
Methods
Seven hundred and forty-nine patients were analyzed. Significant variables were weighted to develop a scoring system for HCC prediction. The area under receiver operating curves (AUROC) were estimated and validated by REVEAL-HBV cohort (n = 3527).
Results
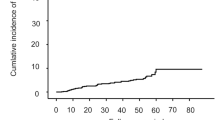
Older age (p < 0.001), male sex (p = 0.036), family history of HCC (p = 0.002) and HBV DNA ≥ 2000 IU/ml (p = 0.045) were independently associated with HCC. A 14-point risk score system predicts 3 and 5-years HCC risk to be 0.866 and 0.868 of AUROC, respectively in the derivation cohort; 0.821 and 0.820, in the REVEAL-HBV cohort. The cumulative HCC incidence was higher in the high risk (score ≥ 8) group both in derivation and validation cohorts (p < 0.001). Patients with antiviral therapy had lower HCC incidence compared to those without (p = 0.016). Of note, antiviral therapy significantly decreased HCC in the high risk group (p = 0.005), but not in the low risk group (p = 0.705).
Conclusions
A risk scoring system is established and validated. Of CHB patients in gray zone of APASL guidelines, those with risk scores ≥ 8 had higher risk of HCC, but the risk could be significantly reduced by antiviral therapy.



Similar content being viewed by others
Data availability
Available.
Code availability
Not applicable.
Change history
22 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12072-023-10634-6
Abbreviations
- ADV :
-
Adefovir
- AFP :
-
Alpha-fetoprotein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CHB:
-
Chronic hepatitis B
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- DFS:
-
Disease free survival
- ER:
-
Endoplasmic reticulum
- ETV:
-
Entecavir
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HBV–HCC:
-
HBV related HCC
- HCC:
-
Hepatocellular carcinoma
- HDL-C:
-
High-density lipoprotein cholesterol
- HR:
-
Hazard ratio
- LAM:
-
Lamivudine
- LDL-C:
-
Low-density lipoprotein cholesterol
- Ldt:
-
Telbivudine
- MRI:
-
Magnetic resonance imaging
- NA:
-
Nucleos(t)ide analogues
- TAF:
-
Tenofovir alafenamide
- TDF:
-
Tenofovir disoproxil fumarate
- qHBsAg:
-
Quantitative hepatitis B surface antigen
References
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555
Wu CY, Lin JT, Ho HJ, Su CW, Lee TY, Wang SY, et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology. 2014;147:143-151e145
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL. Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;2017(67):370–398
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98
Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376–1384
Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47:760–767
Ikeda K, Arase Y, Saitoh S, Kobayashi M, Someya T, Hosaka T, et al. Long-term outcome of HBV carriers with negative HBe antigen and normal aminotransferase. Am J Med. 2006;119:977–985
Chu CM, Liaw YF. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol. 2009;104:1693–1699
Wong VW, Chan SL, Mo F, Chan TC, Loong HH, Wong GL, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28:1660–1665
Yang HI, Yuen MF, Chan HLY, Han KH, Chen PJ, Kim DY, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568–574
Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50:80–88
Sharma SA, Kowgier M, Hansen BE, Brouwer WP, Maan R, Wong D, et al. Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J Hepatol. 2017;68(1):92–99
Hsu YC, Yip TC, Ho HJ, Wong VW, Huang YT, El-Serag HB, et al. Development of a scoring system to predict hepatocellular carcinoma in Asians on antivirals for chronic hepatitis B. J Hepatol. 2018;69:278–285
Kim JH, Kim YD, Lee M, Jun BG, Kim TS, Suk KT, et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol. 2018;69:1066–1073
Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800–806
Wong GL, Chan HL, Wong CK, Leung C, Chan CY, Ho PP, et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol. 2014;60:339–345
Lee HW, Yoo EJ, Kim BK, Kim SU, Park JY, Kim DY, et al. Prediction of development of liver-related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. Am J Gastroenterol. 2014;109:1241–1249
Chon HY, Lee HA, Suh SJ, Lee JI, Kim BS, Kim IH, et al. Addition of liver stiffness enhances the predictive accuracy of the PAGE-B model for hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;53:919–927
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236
Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73
Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063
Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141:1240–1248
Kim S, Lee Y, Bang SM, Bak H, Yim SY, Lee YS, et al. Early normalization of alanine aminotransferase during antiviral therapy reduces risk of hepatocellular carcinoma in HBV patients. J Clin Med. 2021;10(9):1840
Nguyen MH, Garcia RT, Trinh HN, Lam KD, Weiss G, Nguyen HA, et al. Histological disease in Asian-Americans with chronic hepatitis B, high hepatitis B virus DNA, and normal alanine aminotransferase levels. Am J Gastroenterol. 2009;104:2206–2213
Sinn DH, Kim SE, Kim BK, Kim JH, Choi MS. The risk of hepatocellular carcinoma among chronic hepatitis B virus-infected patients outside current treatment criteria. J Viral Hepat. 2019;26:1465–1472
Drafting Committee for Hepatitis Management G, the Japan Society of H. JSH guidelines for the management of hepatitis B virus infection. Hepatol Res. 2014;44(S1):1–58
Kim GA, Han S, Choi GH, Choi J, Lim YS. Moderate levels of serum hepatitis B virus DNA are associated with the highest risk of hepatocellular carcinoma in chronic hepatitis B patients. Aliment Pharmacol Ther. 2020;51:1169–1179
Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531
Liang YJ, Teng W, Chen CL, Sun CP, Teng RD, Huang YH, et al. Clinical implications of HBV PreS/S mutations and the effects of PreS2 deletion on mitochondria, liver fibrosis, and cancer development. Hepatology. 2021;74:641–655
Lazarevic I, Banko A, Miljanovic D, Cupic M. Immune-escape hepatitis B virus mutations associated with viral reactivation upon immunosuppression. Viruses. 2019;11(9):778. https://doi.org/10.3390/v11090778
Loomba R, Liu J, Yang HI, Lee MH, Lu SN, Wang LY, et al. Synergistic effects of family history of hepatocellular carcinoma and hepatitis B virus infection on risk for incident hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2013;11(1636–1645):e1631-1633
Hassan MM, Spitz MR, Thomas MB, Curley SA, Patt YZ, Vauthey JN, et al. The association of family history of liver cancer with hepatocellular carcinoma: a case–control study in the United States. J Hepatol. 2009;50:334–341
Yu MW, Chang HC, Chen PJ, Liu CJ, Liaw YF, Lin SM, et al. Increased risk for hepatitis B-related liver cirrhosis in relatives of patients with hepatocellular carcinoma in northern Taiwan. Int J Epidemiol. 2002;31:1008–1015
Chiba T, Suzuki E, Saito T, Ogasawara S, Ooka Y, Tawada A, et al. Biological features and biomarkers in hepatocellular carcinoma. World J Hepatol. 2015;7:2020–2028
Funding
This study was supported by funding from Ministry of Science and Technology (National Science and Technology Council) (104-2314-B-182A-048-MY3; 110-2314-B-A49A-505; 110-2314-B-075-010-MY3), VGHUST Joint Research Program, Tsou Foundation (VGHUST110-G7-3-3), VGHTPE Research Program (V107C-161, V108C-059 and V109C-049), Ministry of Health and Welfare (MOHW107-TDU-B-211-114019; 108-TDU-B-211-124019; 109-TDU-B-211-134019; 110-TDU-B-211-144019) and Cancer Progression Research Center, National Yang-Ming University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education.
Author information
Authors and Affiliations
Contributions
Concept and design: Wu JC, Su CW; Data acquisition: Wu JC, Su CW, Teng W, Chang TT, Peng CY, Su TH, Yang HC, Yu ML, Hu TH, Yang HI; Manuscript writing: Teng W and Wu JC, Statistical analysis: Teng W, Yang HI, Su CW, Wu JC; Manuscript supervision: Su CW, Wu JC.
Corresponding author
Ethics declarations
Conflicts of interest
Wei Teng, Ting‑Tsung Chang, Hwai‑I Yang, Cheng‑Yuan Peng, Chien‑Wei Su, Tung‑Hung Su, Tsung‑Hui Hu, Ming‑Lung Yu, Hung‑Chih Yang, Jaw‑Ching Wu all authors declared no conflict of interest.
Ethics approval
The study was approved by the Institutional Review Board of each of the participating institutes.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Funding note updated.
Supplementary Information
Below is the link to the electronic supplementary material.




Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teng, W., Chang, TT., Yang, HI. et al. Risk scores to predict HCC and the benefits of antiviral therapy for CHB patients in gray zone of treatment guidelines. Hepatol Int 15, 1421–1430 (2021). https://doi.org/10.1007/s12072-021-10263-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10263-x