Abstract
Background and aims
One-third of the global hepatitis C virus (HCV) burden is found in Asia. Real-world data from diverse East Asian cohorts remain limited. This study addressed the real-world status of direct-acting antiviral (DAA) therapy among patients from East Asia.
Methods
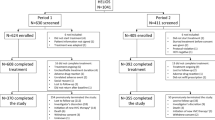
Chronic hepatitis C (CHC) patients from clinical sites in Japan, Taiwan, South Korea, and Hong Kong were recruited in the REAL-C registry, an observational chart review registry. The primary outcome was sustained virologic response (SVR12, HCV RNA PCR < 25 IU/mL 12 week post-therapy).
Results
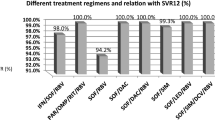
A total of 6287 CHC patients were enrolled. Compared to other East Asian patients, patients from Japan were older (66.3 vs. 61.5 years, p < 0.0001), had lower body mass indices (22.9 kg/m2 vs. 24.6 kg/m2, p < 0.001), and were more likely to have non-liver malignancy history (12.2% vs. 5.0%, p < 0.001).The overall SVR12 rate was 96.4%, similar to patients both inside and outside Japan (96.6% vs. 96%, p = 0.21). The SVR12 rate ranged from 91.1 to 99.4% except treatment-experienced cirrhotic HCV genotype-1 patients who received daclatasvir/asunaprevir (85.9%) and the treatment-experienced cirrhotic HCV genotype-2 patients treated with sofosbuvir/ribavirin (87%). The overall rate of drug discontinuation was 1.9%, also similar across regions. On multivariate regression analyses, there was no significant association between geographic region and SVR outcomes.
Conclusions
In this large multinational CHC cohort from the East Asia, oral DAAs were highly effective and well tolerated across the region. Policies should encourage treatment for all CHC patients with DAAs in Asia with its heavy burden of HCV.



Similar content being viewed by others
Abbreviations
- HCV:
-
Hepatitis C virus
- CHC:
-
Chronic hepatitis C
- HCC:
-
Hepatocellular carcinoma
- DAAs:
-
Direct-acting antivirals
- SVR:
-
Sustained virologic response
- GT:
-
Genotype
- ORs:
-
Odds ratios
- CI:
-
Confidence interval
- LDV:
-
Ledipasvir
- SOF:
-
Sofosbuvir
- RBV:
-
Ribavirin
- DCV:
-
Daclatasvir
- ASV:
-
Asunaprevir
- PrOD:
-
Paritaprevir/ritonavir/ombitasvir/dasabuvir
- EBR:
-
Elbasvir
- GZR:
-
Grazoprevir
- GLE:
-
Glecaprevir
- PIB:
-
Pibrentasvir
- VEL:
-
Velpatasvir
References
The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–76.
Huang CF, Yu ML. Treating hepatitis C in the elderly: pharmacotherapeutic considerations and developments. Exp Opin Pharmacother. 2017;18:1867–74.
Nguyen LH, Nguyen MH. Systematic review: Asian patients with chronic hepatitis C infection. Aliment Pharmacol Ther. 2013;37:921–36.
Lim SG, Aghemo A, Chen PJ, Dan YY, Gane E, Gani R, et al. Management of hepatitis C virus infection in the Asia–Pacific region: an update. Lancet Gastroenterol Hepatol. 2017;2:52–62.
Kao JH, Ahn SH, Chien RN, Cho M, Chuang WL, Jeong SH, et al. Urgency to treat patients with chronic hepatitis C in Asia. J Gastroenterol Hepatol. 2017;32:966–74.
Yu ML, Hepatitis C. Treatment From “Response-guided” to “Resource-guided” therapy in the transition era from IFN-containing to IFN-free regimens. J Gastroenterol Hepatol. 2017;32:1436–42.
Hospital Authority Drug Formulart (HADF). Supplementary operation guideline version 14.1 w.e.f. 14 Jul 2018. 2018 (Hong Kong).
https://www.jshorjp/files/uploads/HCV_GL_ver6_Dec13.pdf Accessed on 20 Sep 2018.
http://www.kaslorg/guideline/guideline_Intro.html Available on 1 Sep 2018.
https://www.nhi.gov.tw/Content_List.aspx?n=A4EFF6CD1C4891CA&topn=3FC7D09599D25979. Accessed on 22 Feb 2019.
Wei B, Ji F, Yeo YH, Ogawa E, Zou B, Stave CD, et al. Real-world effectiveness of sofosbuvir plus ribavirin for chronic hepatitis C genotype 2 in Asia: a systematic review and meta-analysis. BMJ Open Gastroenterol. 2018;5:e000207.
Ji F, Wei B, Yeo YH, Ogawa E. Systematic review with meta-analysis: effectiveness and tolerability of interferon-free direct-acting antiviral regimens for chronic hepatitis C genotype 1 in routine clinical practice in Asia. Aliment Pharmacol Ther. 2018;47:550–62.
Wei B, Ji F, Yeo YH, Ogawa E, Stave CD, Dang S, et al. Systematic review and meta-analysis: real-world effectiveness of direct-acting antiviral therapies in chronic hepatitis C genotype 3 in Asia. BMJ Open Gastroenterol. 2018;5:e000209.
Prenner SB, VanWagner LB, Flamm SL, Salem R, Lewandowski RJ, Kulik L. Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. J Hepatol. 2017;66:1173–81.
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–50.
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol int. 2017;11:317–70.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70.
Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–80.
VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74.
Dumortier J, Bailly F, Pageaux GP, Vallet-Pichard A, Radenne S, Habersetzer F, et al. Sofosbuvir-based antiviral therapy in hepatitis C virus patients with severe renal failure. Nephr Dial Transpl. 2017;32:2065–71.
Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hep Int. 2016;10:702–26.
European Association for The Study of The Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511.
Hellard M, Sacks-Davis R, Doyle J. Hepatitis C elimination by 2030 through treatment and prevention: think global, act in local networks. J Epidemiol Comm Health. 2016;70:1151–4.
Welzel TM, Nelson DR, Morelli G, Di Bisceglie A, Reddy RK, Kuo A, et al. Effectiveness and safety of sofosbuvir plus ribavirin for the treatment of HCV genotype 2 infection: results of the real-world, clinical practice HCV-TARGET study. Gut. 2017;66:1844–52.
Tacke F, Gunther R, Buggisch P, Klinker H, Schober A, John C, et al. Treatment of HCV genotype 2 with sofosbuvir and ribavirin results in lower sustained virological response rates in real life than expected from clinical trials. Liver int. 2017;37:205–11.
Kao JH, Chien RN, Chang TT, Peng CY, Hu TH, Lo GH, et al. A phase 3b study of sofosbuvir plus ribavirin in Taiwanese patients with chronic genotype 2 hepatitis C virus infection. Liver int. 2016;36:1101–7.
Kim YM, Kim SB, Song IH, Lee SH, Kim HS, Lee TH, et al. Efficacy and safety of sofosbuvir plus ribavirin for Korean patients with hepatitis C virus genotype 2 infection: a retrospective multi-institutional study. Clin Mol Hepatol. 2018;24:311–8.
Acknowledgements
The authors gratefully acknowledge the contributions of the study coordinators and staff from all participating institutions who assisted in the data collection and other logistics for this study.
For the REAL-C Investigators: Sang Bong Ahn: Department of Internal Medicine, Eulji University Seoul Hospital, Seoul, South Korea; Koichi Azuma: Department of Medicine, Kyushu Central Hospital, Fukuoka, Japan; Tzu-Haw Chen: Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan; Chia-Yen Dai: Hepatobiliary Division, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan; Kazufumi Dohmen: Department of Internal Medicine, Chihaya Hospital, Fukuoka, Japan; Mi Jung Jun: Department of Gastroenterology, Good Gang-An Hospital, Busan, Korea; Jang Han Jung: Department of Internal Medicine, Hallym University Dongtan Sacred Heart Hospital, Hwaseong-si, South Korea; Eiji Kajiwara: Kajiwara Clinic, Kitakyushu, Japan; Masaki Kato: Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan; Akira Kawano: Department of Medicine, Kitakyushu Municipal Medical Center, Kitakyushu, Japan; Toshimasa Koyanagi: Department of Medicine, Fukuoka City Hospital, Fukuoka, Japan; Aritsune Ooho: Department of Hepatology, Steel Memorial Yawata Hospital, Kitakyushu, Japan; Takeaki Satoh: Center for Liver Disease, National Hospital Organization Kokura Medical Center, Kitakyushu, Japan; Shinji Shimoda: Department of Medicine and Biosystemic Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan; Do Seon Song: Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, South Korea; Hirokazu Takahashi: Liver Center, Saga University Hospital, Saga, Japan; Division of Metabolism and Endocrinology, Saga University Faculty of Medicine, Saga, Japan; Kazuhiro Takahashi: Department of Medicine, Hamanomachi Hospital, Fukuoka, Japan; Pei-Chien Tsai: Hepatobiliary Division, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan; Chao-Ming Tseng: Division of Gastroenterology and Hepatology, Department of Internal Medicine, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan; Ming-Lun Yeh: Hepatobiliary Division, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan; Eileen L. Yoon: Department of Internal Medicine, Inje University Sanggye Paik Hospital, Seoul, South Korea—are in alphabetical order.
Funding
This study is supported by an investigator-initiated research grant (IN-US-334-4309) from Gilead Sciences to Stanford University. The authors independently collected data, designed and performed all study analyses, and drafted the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
Guarantor of the study: NMH. All authors: study design and/or data collection, data analysis and/or interpretation, and critical review and/or revision of the manuscript. Drafting of the manuscript: HCF, TS, KL, HL, CR, MLY, and NMH. Study concept, acquisition of funding, and study supervision: NMH
Corresponding author
Ethics declarations
Conflict of interest
HT: Speaker for AbbVie and MSD. YCH: Research support from Gilead; advisory board for Gilead; speaker for Abbvie, BMS, Gilead, and Merck Sharp & Dohme. GW: Research support from Gilead; advisory board/consulting for Gilead; speaker for Abbott, Abbvie, BMS, Echosens, Furui, and Gilead. CHL: Research support from Abbvie, Gilead, and MSD; advisory board/consulting for Abbvie, Gilead, and MSD; speaker for Abbvie, Gilead, MSD, and Abbott. WLC: Advisory board for Gilead, Abbvie, MSD, BMS, PharmaEssentia. RC: Research support from Gilead. CYD: Advisory board for Abbvie; speaker for Abbvie, Merck, and Gilead. JHK: Research support from Gilead and BMS; advisory board/consulting for Gilead, Abbvie, BMS, and MSD; speaker for Gilead, Abbvie, MSD, and BMS. YU: Research support from Abbvie, Gilead, and Bayer. NF: Research support from Janssen Pharmaceutical K.K., Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and Abbvie GK; speaker for Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, Torii Pharmaceutical Co., Ltd. and Roche Diagnostics K.K. MLY: Research support: Abbvie, BMS, Gilead, Torpedo and Merck; consultant for Abbvie, Abbott, Ascletis, BMS, Gilead, Merck, and PharmaEssentia; speaker for Abbvie, Abbott, Ascletis, BMS, Gilead, and Merck. YT: Research support from Chugai Pharmaceutical, Janssen, and the Japan Agency for Medical Research and Development (AMED); honoraria from Gilead. MHN: Research support from Janssen, Pfizer, Gilead, National Cancer Institute; BK Kee Foundation; advisory board/consulting for Novartis, Gilead, Janssen, Bayer, Eisai, Exact Sciences, Laboratory of Advanced Medicine. All other authors have no relevant conflict of interests to disclose.
Ethical approval
The study was conducted according to the Helsinki Declaration of 1975, as revised in 2008, and was approved by the Institutional Review Board at Stanford University, Stanford, California, USA and participating study centers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, CF., Iio, E., Jun, D.W. et al. Direct-acting antivirals in East Asian hepatitis C patients: real-world experience from the REAL-C Consortium. Hepatol Int 13, 587–598 (2019). https://doi.org/10.1007/s12072-019-09974-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-09974-z