Abstract
Background/purpose
Lenvatinib (an inhibitor of vascular endothelial growth factor (GF) receptors 1–3, fibroblast GF receptors 1–4, platelet-derived GF receptor α, rearranged during transfection, and stem cell factor receptor) was non-inferior to sorafenib in a phase 3 (REFLECT) trial of advanced hepatocellular carcinoma. This study examined the efficacy and safety of lenvatinib in a real-world setting.
Methods
This was a retrospective, multicenter, observational study. Inclusion and exclusion criteria were based on the phase 3 trial, and participants were observed for at least 12 weeks. Therapeutic effect was determined using the modified Response Evaluation Criteria In Solid Tumors (m-RECIST) at the 8th week. Patients received oral lenvatinib 12 mg/day (body weight > 60 kg) or 8 mg/day (body weight < 60 kg). Dose interruptions followed by reductions for lenvatinib-related toxicities were permitted. Grades of adverse events (AEs) complied with the Common Terminology Criteria for Adverse Events version 4.0.
Results
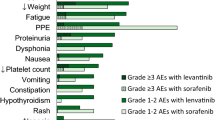
All 16 patients included in this study had prior treatment history, and a median 3.9 years had passed since the first treatment. Fatigue, hypertension, and proteinuria were the most frequent AEs, and were higher than Grade 2. AEs could be controlled by appropriate dose reduction, interruption, and symptomatic treatment according to the protocol. In the m-RECIST evaluation at the 8th week, 0, 6, 8, and 1 patients had achieved complete response, partial response, stable disease, and progressive disease, respectively. The objective response rate was 40%.
Conclusion
Lenvatinib treatment could be accomplished with safety and good response in a real-world setting.


Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386
Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016;150:835–853
Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M et al . Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017;67:302–309
Cabibbo G, Maida M, Genco C, Parisi P, Peralta M, Antonucci M et al. Natural history of untreatable hepatocellular carcinoma: a retrospective cohort study. World J Hepatol 2012;4:256–261
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317–370
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, SHARP Investigators Study Group et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–390
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34
Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol 2013;31:4067–4075
Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013;31:3517–3524
Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol 2015;33:172–179
Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015;33:559–566
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–1173
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52–60
Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329–338
Child CG, Turcotte JG. Surgery and Portal Hypertension. The Liver and Portal Hypertension. Philadelphia: Saunders; 1964. 50–64.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–649
Oken M, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–655
Common Terminology Criteria for Adverse Events v4.0 (CTCAE) May 28, 2009 NIH Publication No. 03-5410. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50
Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Itobayashi E, Shimada N, et al. Real-life Practice Experts for HCC (RELPEC) Study Group and the HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: multicenter analysis. Hepatol Res 2018. https://doi.org/10.1111/hepr (Epub 2018 Aug 24)
Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621–630
Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol 2017;52:512–519
Boss DS, Glen H, Beijnen JH, Keesen M, Morrison R, Tait B et al. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br J Cancer 2012;106:1598–1604
Tanaka K, Shimada M, Kudo M. Characteristics of long-term survivors following sorafenib treatment for advanced hepatocellular carcinoma: report of a workshop at the 50th Annual Meeting of the Liver Cancer Study Group of Japan. Oncology 2014;87(Suppl 1):104–109
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for being included in the study. All relevant institutional review boards approved the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Obi, S., Sato, T., Sato, S. et al. The efficacy and safety of lenvatinib for advanced hepatocellular carcinoma in a real-world setting. Hepatol Int 13, 199–204 (2019). https://doi.org/10.1007/s12072-019-09929-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-09929-4