Abstract
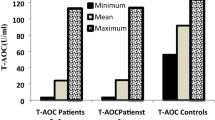
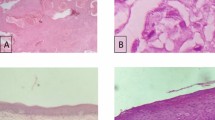
Antioxidants are widely used in chemoprevention of malignancy. Numerous studies in medical literature have reported the evaluation of this treatment protocol by indirect methodology—epidemiology, invitro studies, pharmacology and animal models etc. However, there is a paucity of literature on the measurement of antioxidant enzymes as a parameter for assessing the outcome of antioxidant therapy. This study explores the efficacy and outcome of antioxidant enzyme assay in relation to antioxidant therapy in tobacco abusers, hitherto unreported in medical literature. A prospective cohort study with control in 50 patients carried out at a tertiary care teaching Institution (Institute of Medical Sciences, Banaras Hindu University, Varanasi, India). Out of these patients, 10 patients acted as control, rest 40 patients—all tobacco users in some form, were divided into three groups on the basis of histopathological grading of dysplasia—no dysplasia, mild or moderate dysplasia. The levels of Lipid peroxidase (LPO), Superoxide dismutase (SOD) and Catalase (CAT) in mucosa and serum were assayed in each group, and re-evaluated at the end of 3 months after intervention with antioxidant treatment. To detect any alteration in degree of dysplasia a repeat biopsy was also done at the end of 3 months. The results were statistically analysed using paired t test. A statistically significant decrease in level of LPO and SOD, and an increase in CAT levels were recorded both in mucosa and serum. However, no change in dysplasia and no new case of dysplasia were observed. Further, antioxidant treatment was continued for a year and the final out come of the lesion was assessed by “Carter’s criteria”. A final success rate of 74.19% was recorded in terms of partial or complete regression of the lesion. This study confirms the therapeutic efficacy of antioxidants in oral leukoplakia, and cites the importance of LPO, SOD and CAT in evaluating the efficacy of antioxidant treatment. However, the study failed to elucidate any relationship between enzyme measurement and the final outcome of the lesion.
Similar content being viewed by others
References
Singh M, Krishanappa R, Bagewadi A, Keluskar V (2004) Efficacy of oral lycopene in the treatment of oral leukoplakia. Oral Oncol 40:591–596
Fali S, Mehta (1999) Text book of tobacco related oral mucosal lesions and conditions in India. Dental Research Unit, TATA Institute of Fundamental Research, Bombay, p 10
Pindborg J (1980) Text book of oral cancer and pre-cancer. John Wright & Sons Ltd, Bristol, p 45
Kumar V, lingan MW (2004) Head & neck. In: Kumar V, Abbas Ak (eds) Robbins & Cotran pathologic basis of disease, 7th edn. Elsevier, St. Louis, p 778
Singh VN, Gaby KS (1991) Premalignant lesions: role of antioxidant vitamins and beta-carotene in risk reduction and prevention of malignant transformation. Am J Clin Nutr 53:386S–390S
Garewal H (1995) Antioxidants in oral cancer prevention. Am J Clin Nutr 62(6 Suppl):1410S–1416S
Subapriya R, Kumaraguruparan R, Ramachandran CR, Nagini S (2002) Oxidant–antioxidant status in patients with oral squamous cell carcinoma at different interoral sites. Clin Biochem 35(6):489–493
Yang J, Lam EW, Hammad HM, Oberley TD, Oberley LW (2002) Antioxidant enzyme levels in oral squamous cell carcinoma and normal human oral epithelium. J Oral Pathol Med 31(2):71–77
Patel BP, Rawal UM, Shah PM, Prajapati JA, Rawal RM, Dave TK et al (2005) Study of tobacco habits and alterations in enzymatic antioxidant system in oral cancer. Oncology 68(4–6):511–519
Manoharan S, Kolanjiappan K, Suresh K, Panjamurthy K (2005) Lipid peroxidation & antioxidants status in patients with oral squamous cell carcinoma. Indian J Med Res 122:529–534
kakkar P, Das B, Vishwanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Aebi H, Wyss SR, Scherz B, Skavil F (1974) Heterogeneity of erythrocyte Catalase II. Isolation and characterization of normal and variant erythrocyte Catalase and their subjuncts. Eur J Biochem 48(1):137–145
Ohkaura H, Ohishi N, Yagi K (1979) Assay for lipid peroxidase in animal tissue by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Halliwell B (1996) Mechanisms involved in the generation of free radicals. Pathol Biol 44:6–13
Kehrer JP (1993) Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 23:21–48
Abidi S, Ali A (1999) Role of oxygen free radicals in the pathogenesis and etiology of cancer. Cancer Lett 142:1–9
VanGinkel G, Sevanian A (1994) Lipid peroxidation-induced membrane structural alterations. Methods Enzymol 233:273–288
Manoharan S, Shreeram S, Nagini S (1996) Life style can induce lipid peroxidation and compromise of antioxidant defence mechanism in the erythrocytes of oral cancer patients. Med Sci Res 24:397–400
Manoharan S, Nagini S (1994) Lipid peroxidation and antioxidant status in oral cancer patients. Med Sci Res 22:291–292
Krinsky NI, Deneke SM (1982) The interaction of oxygen and oxy-radicals with carotenoids. J Natl Cancer Inst 69:205–210
Mobarrhan S, Bowen P, Andersen B, Evans M, Stacewic Z, Sugarman S et al (1990) Effects of beta carotene repletion on beta carotene absorption, lipid peroxidation, and neutrophil superoxide formation in young men. Nutr Cancer 14(3–47):195–206
Fridovich I (1976) Oxygen radicals, hydrogen peroxide and oxygen toxicity. In: Pryor WA (ed) Free radicals in biology. Academic Press, New York, p 239
Touti D (1988) Molecular genetics of superoxide dismutases. Free Radic Biol Med 5:393–402
landriscine M, Remidddi F, Ria F, Palazzoti B, de Leo ME, Lacoangeli M et al (1996) The level of MnSOD is directly correlated with grade of brain tumours of neuroepithelial origin. Br J Cancer 74:1877–1885
Sun Y (1990) Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med 8:583–599
Mates JM, Perez-Gomez C, de Numez castro I (1999) Antioxidant enzymes and human diseases. Clin Biochem 32:595–603
Garewal HS, Katz RV, Meyskens F, Pitcock J, Morse D, Friedman S et al (1999) Beta carotene produces sustained remissions in patients with oral leukoplakia. Arch Otolaryngol Head Neck Surg 125:1305–1310
Krishnaswamy K, Prasad MPR, Krishna TP, Annapurna VV, Reddy GA (1995) A case study of nutrient intervention of oral precancerous lesions in India. Oral Oncol 31B:41–48
Stich HF, Rosin MP, Hornby AP (1988) Remission of oral leukoplakias and micronuclei in tobacco/betal chewers treated with beta-carotene and with beta-carotene plus vitamin A. Int J Cancer 4:199
Kaugars GE, Silverman S, Lovas JGL (1994) A clinical trial of antioxidant supplements in the treatment of oral leukoplakia. Oral surg Oral Med Pathol Oral Radiol Endod 78:462–468
Maleker K, Anderson BJ, Beecroft WA, Hodson DI (1991) Management of oral mucosal dysplasia with carotene retinoic acid: a pilot cross-over study. Cancer Detect Prev 15:335–340
Garewal HS, Schantz S (1995) Emerging role of beta-carotene and antioxidant nutrients in prevention of oral cancer. Arch Otolaryngol Head Neck Surg 121:141–144
Pillai RK, Garewal HS, Wood S, Watson RR (1992) Biological monitoring of cancer chemoprevention. J Surg Oncol 51:195–202
Benner SE, Winn RW, Lippman SM et al (1993) Regression of oral leukoplakia with alpha-tocopherol: a community clinical oncology program chemoprevention study. J Natl Cancer Inst 85:44–47
Gaby SK, Singh VN (1991) Beta-carotene. In: Gaby Sk, Bendich A, Singh VN, Achin LJ (eds) Vitamin intake and health. Marcel Dekker, New York, pp 29–57
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jain, R.K., Singh, G.B., Singh, A.P. et al. Role of Measurement of Antioxidant Enzymes in Evaluation of Antioxidant Therapy in Tobacco Abusers with Oral Leukoplakia. Indian J Otolaryngol Head Neck Surg 63, 336–342 (2011). https://doi.org/10.1007/s12070-011-0266-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-011-0266-y