Abstract
Purpose
There is increasing clinical utilization of hearts from the donation after circulatory death (DCD) pathway with the aim of expanding the donor pool and mitigating the ever-present discrepancy between the inadequate availability of good quality donor hearts and the rising number of patients with end-stage heart failure.
Methods
This article reviews the rationale, practice, logistical factors, and 5-year experience of DCD heart transplantation at St Vincent’s Hospital, Sydney.
Findings
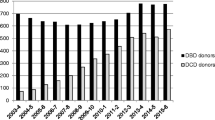
Between July 2014 and July 2019, 69 DCD donor retrievals were undertaken resulting in 49 hearts being instrumented on an ex situ normothermic cardiac perfusion device. Seventeen (35%) of these hearts were declined and the remaining 32 (65%) were used for orthotopic DCD heart transplantation. At 5 years of follow-up, the 1-, 3-, and 5-year survival was 96%, 94%, and 94% for DCD hearts compared with 89%, 83%, and 82% respectively for donation after brain death (DBD) hearts (n.s). The immediate post-implant requirement for temporary extra-corporeal membrane oxygenation (ECMO) support for delayed graft function was 31% with no difference in rejection rates when compared with the contemporaneous cohort of patients transplanted with standard criteria DBD hearts.
Summary
DCD heart transplantation has become routine and incorporated into standard clinical practice by a handful of pioneering clinical transplant centres. The Australian experience demonstrates that excellent medium-term outcomes are achievable from the use of DCD hearts. These outcomes are consistent across the other centres and consequently favour a more rapid and wider uptake of heart transplantation using DCD donor hearts, which would otherwise be discarded.



Similar content being viewed by others
References
Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385:2585–91.
Barnard CN. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967;41:1271–4.
Boucek MM, Mashburn C, Dunn SM, et al. Pediatric heart transplantation after declaration of cardiocirculatory death. N Engl J Med. 2008;359:709–14.
Kleinmahon JA, Patel SS, Auerbach SR, et al. Hearts transplanted after circulatory death in children: Analysis of the International Society for Heart and Lung Transplantation registry. Pediatr Transplant. 2017;21:e13064.
Messer S, Large S. Resuscitating heart transplantation: the donation after circulatory determined death donor. Eur J Cardiothorac Surg. 2016;49:1–4.
Garcia Saez D, Bowles CT, Mohite PN, et al. Heart transplantation after donor circulatory death in patients bridged to transplant with implantable left ventricular assist devices. J Heart Lung Transplant. 2016;35:1255–60.
Mehta V, Taylor M, Hasan J, et al. Establishing a heart transplant programme using donation after circulatory-determined death donors: a United Kingdom based single-centre experience. Interact Cardiovasc Thorac Surg. 2019;29:422–9.
http://www.newcastle-hospitals.org.uk/news/news-item-23933.aspx.
https://surgery.duke.edu/news/doctors-duke-university-hospital-perform-first-dcd-heart-transplant-us.
Messer S, Page A, Colah S, et al. Human heart transplantation from donation after circulatory- determined death donors using normothermic regional perfusion and cold storage. J Heart Lung Transplant. 2018;37:865–9.
Kushnood A, Butt TA, Jungschleger J, et al. Paediatric donation after circulatory determined death heart transplantation using donor normothermic regional perfusion and ex situ heart perfusion: A case report. Pediatr Transplant. 2019;23:e13536.
Tchana-Sato V, Ledoux D, Detry O, et al. Successful clinical transplantation of hearts donated after circulatory death using normothermic regional perfusion. J Heart Lung Transplant. 2019;38:593–8.
Tchana-Sato V, Ledoux D, Vandendriessche K, et al. First report of successful pediatric heart transplantation from donation after circulatory death with distant procurement using normothermic regional perfusion and cold storage. J Heart Lung Transplant. 2019;38:1112–5.
Lomero M, Gardiner D, Coli E, et al. Donation after circulatory death today: an updated overview of the European landscape. Transpl Int. 2020;33:76–88.
Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int. 2016;29:749–59.
Bollen JAM, Shaw D, de Wert G, et al. Euthanasia through living organ donation: ethical, legal, and medical challenges. J Heart Lung Transplant. 2019;38:111–3.
Watson A, Gao L, Sun L, et al. Enhanced preservation of pig cardiac allografts by combining erythropoietin with glyceryl trinitrate and zoniporide. Am J Transplant. 2013;13:1676–87.
Iyer A, Gao L, Doyle A, et al. Increasing the tolerance of DCD hearts to warm ischemia by pharmacological postconditioning. Am J Transplant. 2014;14:1744–52.
Iyer A, Gao L, Doyle A, et al. Normothermic ex vivo perfusion provides superior organ preservation and enables viability assessment of hearts from DCD donors. Am J Transplant. 2015;15:371–80.
Iyer A, Chew HC, Gao L, et al. Pathophysiological trends during withdrawal of life support: implications for organ donation after circulatory death. Transplantation. 2016;100:2621–9.
Iyer A, Wan B, Kumarasinghe G, et al. What is the potential source of heart allografts from donation after circulatory death (DCD) donors? Transplantation. 2013;96:S217.
Listijono DR, Watson A, Pye R, et al. Usefulness of extracorporeal membrane oxygenation for early cardiac allograft dysfunction. J Heart Lung Transplant. 2011;30:783–9.
https://donatelife.gov.au/sites/default/files/Brain_Death_Determination_Statement.pdf.
Cao Y, Shahrestani S, Chew H, et al. Donation after circulatory death for liver transplantation: A meta-analysis on the location of life support withdrawal affecting outcomes. Transplantation. 2016;100:1513–24.
Connellan M, Dhital K. Donor heart procurement from the donation after circulatory death pathway. Oper Tech Thorac Cardiovasc Surg. 2017;22:58–67.
Chew HC, Iyer A, Connellan M, et al. Outcomes of donation after circulatory death heart transplantation in Australia. J Am Coll Cardiol. 2019;73:1447–59.
Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischaemic preconditioning. AM J Physiol Heart Circ Physiol. 2003;285:H579–88.
Hausenloy DJ, Barrabes JA, Botker HE, et al. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol. 2016;111:70.
Gao L, Hicks M, Macdonald PS. Improved preservation of the rat heart with Celsior solution supplemented with cariporide plus glyceryl trinitrite. Am J Transplant. 2005;5:1820–6.
Hing AJ, Watson A, Hicks M, et al. Combining cariporide with glyceryl trinitrite optimizes cardiac preservation during porcine heart transplantation. Am J Transplant. 2009;9:2048–56.
Gao L, Tsun J, Sun L, et al. Critical role of the STAT 3 pathway in the cardioprotective efficacy of zoniporide in a model of myocardial preservation- The rat isolated working heart. Br J Pharmacol. 2011;162:633–47.
Watson AJ, Gao L, Sun L, et al. Enhanced preservation of the rat heart after prolonged hypothermic ischaemia with erythropoietin supplemented Celsior solution. J Heart Lung Transplant. 2013;32:633–40.
Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomized, non-inferiority trial. Lancet. 2015;385:2577–84.
Messer S, Ardehali A, Tsui S. Normothermic donor heart perfusion: current clinical experience and the future. Transpl Int. 2015;28:634–42.
Garcia Saez D, Zych B, Sabashnikov A, et al. Evaluation of the organ care system in heart transplantation with adverse donor/recipient profile. Ann Thorac Surg. 2014;98:2099–105.
Connellan M, Chew H, Iyer A, et al. Ex-vivo perfusion of marginal donor hearts: is normal allograft function assured post-transplant? J Heart Lung Transplant. 2019;25:e92.
Sponga S, Ferrara V, Beltrami AP, et al. Ex-vivo perfusion on marginal donors in heart transplantation: Clinical results and pathological findings. J Heart Lung Transplant. 2019;38:s42–3.
Schroder JN, D’Alessandro D, Esmailian F, et al. Successful utilization of extended criteria donor (ECD) hearts for transplantation – results of the OCS heart EXPAND trial to evaluate the effectiveness and safety of the OCS heart system to preserve and assess ECD hearts for transplantation. J Heart Lung Transplant. 2019;38:s42.
Messer S, Page A, Axell R, et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2017;36:1311–8.
Anthony C, Michel J, Christofi M, et al. Ex vivo coronary angiographic evaluation of a beating donor heart. Circulation. 2014;130:e341–3.
No authors listed. A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to examine the definition of brain death. JAMA. 1968;205:337–40.
Machando C, Korein J, Ferrer Y, et al. The declaration of Sydney on human death. J Med Ethics. 2007;33:699–703.
Steen S, Paskevicius A, Liao Q, Sjoberg T. Safe orthotopic transplantation of hearts harvested 24 hours after brain death and preserved for 24 hours. Scand Cardiovasc J. 2016;50:193–200.
Acknowledgements
The authors thank Dr. Helen Opdam, the National Medical Director of the Australian Organ & Tissue Authority, for collating information of the use of ante mortem heparin in the various Australian states.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhital, K., Ludhani, P., Scheuer, S. et al. DCD donations and outcomes of heart transplantation: the Australian experience. Indian J Thorac Cardiovasc Surg 36 (Suppl 2), 224–232 (2020). https://doi.org/10.1007/s12055-020-00998-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-020-00998-x