Abstract
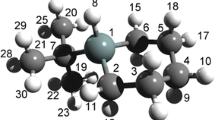
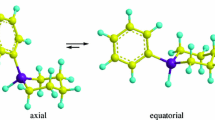
The neutron diffraction data analysis of deuterated liquid 2-propanol at room temperature to define its molecular conformation is presented. 2-Propanol being a large molecule with twelve atomic sites, the conformation analysis is tricky and an improved method of data analysis is given. The intermolecular structural correlations, i.e., hydrogen-bonded liquid structure, can be modelled accurately to extract the nature of the average hydrogen-bonded molecular association in liquid state at room temperature. Like other alcohols these are mostly hexamer ring chain (HRC) clusters. The cluster analysis of recent X-ray data available in the literature also support the same liquid structure.
Similar content being viewed by others
References
S Sarkar and R N Joarder, J. Chem. Phys. 99, 2032 (1993)
S Sarkar and R N Joarder, J. Chem. Phys. 100, 5118 (1994)
A K Karmakar, S Sarkar and R N Joarder, J. Phys. Chem. 99, 16501 (1995)
P P Nath, S Sarkar, P S R Krishna and R N Joarder, Z. Naturforsch A56, 825 (2001)
P P Nath, S Sarkar, P S R Krishna and R N Joarder, Appl. Phys. A74, S348 (2002)
M Magini, G Paschina and G Piccaluga, J. Chem. Phys. 77, 2051 (1982)
I M Svishahov and P G Kusalik, J. Chem. Phys. 100, 5165 (1994)
D T Bowron, J L Finney and A K Soper, Mol. Phys. 93, 531 (1998)
Sidney W Benson, J. Am. Chem. Soc. 118, 10645 (1996)
T Takamuku, K Saisho, S Aoki and T Yamaguchi, Z. Naturforsch A57, 982 (2002)
P Zetterstrom, U Dahlborg, R G Delaplane and W S Howells, Phys. Scr. 44, 56 (1991)
P Zetterstrom, U Dahlborg and W S Howells, Mol. Phys. 81, 1187 (1994)
W Bertagnolli, P Chieux and M D Zeidler, Mol. Phys. 32, 759 (1976)
J Frenkel, Kinetic theory of gases (Dover, New York, 1955)
A H Narteen and A Habenschuss, J. Chem. Phys. 80, 3387 (1984)
V N E Abdel Aziz and F Rogowski, Z. Naturforsch B19, 967 (1964)
A K Karmakar, P S R Krishna and R N Joarder, Phys. Lett. A253, 207 (1999)
A Sahoo, S Sarkar, V Bhagat and R N Joarder, J. Phys. Chem. A113, 5160 (2009)
A K Adya, L Bianchi and C J Wormald, J. Chem. Phys. 112, 4231 (2000)
L Pauling, The nature of chemical bond (Oxford and IBH Publishing Co., New Delhi, 1967) 3rd Ed
S Kashtanov, A Augustson, J E Rubensson, J Nordgren, H Agren, J-H Guo and Y Luo, Phys. Rev. B71, 104205 (2005)
M V Gonzalez, H S Martin, J H Cobos, R Ayala, E S Marcos and I O Blake, J. Chem. Phys. 127, 224507 (2007)
M R Ramanadhan (BARC), Private Communication (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahoo, A., Sarkar, S., Krishna, P.S.R. et al. Molecular conformation and liquid structure of 2-propanol through neutron diffraction. Pramana - J Phys 74, 765–779 (2010). https://doi.org/10.1007/s12043-010-0097-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12043-010-0097-5