Abstract
Mutation and recombination are primarily responsible for generating the genetic variability in natural populations of microorganisms, plant and animal species including humans. Upon such genetic variations, elemental forces of evolution such as natural selection, random genetic drift and migration operate to bring about micro-evolutionary changes. Recombination or crossing-over produces new combinations of genes due to interchange of corresponding segments between nonsister chromatids of homologous chromosomes, thus, it is an important evolutionary factor. Since the time of T. H. Morgan, Drosophila has been subjected to extensive investigations on crossing over while employing a number of markers, which were used for gene mapping. Interestingly, recombination occurs in females of D. melanogaster but not in males. Later on, male crossing over was investigated in various species and its occurrence was reported in D. melanogaster, D. ananassae, D. simulans, D. willistoni, D. littoralis and D. bipectinata. Recombination occurs at very low rate in all these species except for D. ananassae, which shows spontaneous male crossing over in appreciable frequency, which is meiotic in origin. This unusual phenomenon in D. ananassae is influenced by various genetic factors as well as it shows strain variation as far as frequency of male recombination is concerned. Further, the presence of chiasmata during meiosis in males at a frequency capable of accounting for the observed recombination frequency extends evidence for meiotic origin of recombination in males of D. ananassae.

Similar content being viewed by others
References
Friesen H. 1934 Causes of suppression of crossing-over in males of Drosophila melanogaster. Nature 134, 3383.
Friesen H. 1936 Spermatogoniales crossing-over bei Drosophila. Z. induct. Abstamm. U. Vererb.-Lehre 71, 501–526.
Friesen H. 1937 Mechanism of crossing-overt in males of Drosophila melanogaster. J. Genet. 35, 141–150.
Goni B., Matsuda M., Yamamoto M., Vilela C. R. and Tobari Y. N. 2012 Crossing-over does occur in males of Drosophila ananassae from natural populations. Genome 55, 505–511.
Grell R. F., Bank H. and Gassner G. 1972 Meiotic exchange without the synaptonemal complex. Nature 240, 165–167.
Hinton C. W. 1970 Identification of two loci controlling crossing-over in males of Drosophila ananassae. Genetics 66, 663–676.
Hinton C. W. 1974 An extrachromosomal suppressor of male crossing-over in Drosophila ananassae. In Mechanism of recombination (ed. R. F. Grell), pp. 391–397. Plenum Publishing Corporation, New York.
Hinton C. W. 1981 Nucleocytoplasmic relations in a mutator-suppressor system of Drosophila ananassae. Genetics 98, 77–90.
Hinton C. W. 1983 Relation between factors controlling crossing-over and mutability in males of Drosophila ananassae. Genetics 104, 95–112.
Hinton C. W. and Downs J. F. 1975 The mitotic, polytene and meiotic chromosomes of Drosophila ananassae. J. Hered. 66, 353–361.
Kale P. G. 1967a Induced crossing-over in the males of Drosophila ananassae: exchanges mainly in spermatocytes of adults. Mutat. Res. 4, 631–639.
Kale P. G. 1967b Crossing-over in the males of Drosophila ananassae: exchanges in irradiated pupae. Int. J. Rad. Biol. 13, 1–12.
Kale P. G. 1968 Spontaneous crossing-over in the males of Drosophila ananassae: two way selection for recombination values. Jap. J. Genet. 43, 27–31.
Kale P. G. 1969 The meiotic origin of spontaneous crossovers in Drosophila ananassae males. Genetics 62, 123–133.
Kikkawa H. 1938 Studies on the genetics and cytology of Drosophila ananassae. Genetica 20, 458–516.
Mavor J. A. and Svenson H. K. 1924 An effect of X-Rays on the linkage of Mendelian characters in the second chromosome of Drosophila melanogaster. Genetics 9, 70–89.
Matsuda M. 1986 The effect of temperature on recombination in males of Drosophila ananassae. Dros. Inf. Serv. 63, 94.
Matsuda M. and Tobari Y. N. 1982 Influence of male age on male recombination frequency in Drosophila ananassae. Zool. Mag. 91, 1–7.
Matsuda M., Imai H. T. and Tobari Y. N. 1983 Cytogenetic analysis of recombination in males of Drosophila ananassae. Chromosoma 88, 286–289.
Matsuda M., Sato H. and Tobari Y. N. 1993 Crossing-over in males. In Drosophila ananassae: genetical and biological aspects (ed. Y. N. Tobari), pp. 53–72. Japan Scientific Societies Press, Karger.
Mohanty S. and Singh B. N. 1992 Effect of directional selection on spontaneous male recombination in Drosophila ananassae. Ind. J. Exp. Biol. 30, 19–22.
Morgan T. H. 1912 Complete linkage in the second chromosome of the male of Drosophila. Science 36, 719–720.
Morgan T. H. 1914 No crossing over in the male of Drosophila of the genes in the second and third pairs of chromosomes. Biol. Bull. 26, 195–204.
Meyer G. F. 1960 The fine structure of the spermatocyte nuclei of Drosophila melanogaster. Proc. Europ. Region Conf. 2, 951–954.
Moriwaki D. 1937 A high ratio of crossing-over in Drosophila ananassae. Zeit. Induct. Abstammungs-Vererbungsl. 74, 17–23.
Moriwaki D. 1940 Enhanced crossing-over in the second chromosome of Drosophila ananassae. Jap. J. Genet. 16, 37–48.
Moriwaki D. and Tobari Y. N. 1973 Spontaneous male crossing-over of frequent occurrence in Drosophila ananassae from South-East Asian populations. Jap. J. Genet. 48, 167–173.
Moriwaki and Tobari 1975 Drosophila ananassae. In Hand book of genetics (ed. R. C. King), pp. 513–535. Plenum Press, New York.
Moriwaki D. and Tsujita M. 1974 Synaptonemal complex and male crossing-over in Drosophila ananassae. Cytologia 39, 829–838.
Moriwaki D. Tobari Y. N. and Oguma Y. 1970 Spontaneous crossing-over in males of Drosophila ananassae. Jap . J. Genet. 45, 411–420.
Moriwaki D., Tobari Y. N. and Matsuda M. 1979 Role of Y-chromosome in male crossing-over in Drosophila ananassae. Jap. J. Genet. 54, 295–302.
Mukherjee A. S. 1961 Effect of selection on crossing-over in the male of Drosophila ananassae. Am. Nat. 95, 57–59.
Mukherjee A. S. and Das A. 1971 Segregation distorsion and crossing-over in males of Drosophila ananassae. I. Preliminary genetic analysis. Genetics 67, 521–532.
Parker D. R. 1948 Observations on crossing-over induced by X-rays in the males of Drosophila. Genetics 33, 304–310.
Patterson J. and Suche M. L. 1934 Crossing-over induced by X-rays in Drosophila males. Genetics 19, 223–236.
Plough N. H. 1921 Further studies on the effect of temperature on crossing-over. J. Exp. Zool. 32, 178–202.
Ray-Chaudhuri S. P. and Kale P. G. 1966 Crossing-over in the males of Drosophila ananassae. J. Cytol. Genet. (India) 1, 22–29.
Sandler L. and Novitski E. 1957 Meiotic drive as an evolutionary force. Am. Nat. 91, 105–110.
Sandler L., Hiraizumi Y. and Sandler I. 1959 Meiotic drive in natural populations of Drosophila melanogaster I: cytogenetic basis of segregation distortion. Genetics 44, 233–250.
Sato H., Goni B., Matsuda M. and Tobari Y. N. 2000 A site specific increase in recombination in Drosophila ananassae. Genes Genet. Syst. 75, 41–47.
Singh B. N. 1973 Recombination between heterozygous inversions in Drosophila ananassae. Genetica 44, 602–607.
Singh B. N. 1974 On the combinations of different gene arrangements in the third chromosome of Drosophila ananassae. Caryologia 27, 285–292.
Singh B. N. 1985 Drosophila ananassae–a genetically unique species. Nucleus 28, 169–176
Singh B. N. 1994 Chromosomal variability in Drosophila. In Perspectives of entomological research (ed. O. P. Agarwal), pp. 177–188, Scientific Publishers, Jodhpur.
Singh B. N. 1998 Population genetics of inversion polymorphism in Drosophila ananassae. Ind. J. Exp. Biol. 36, 739–748.
Singh B. N. 2000 Drosophila ananassae – a species characterized by several unusual genetic features. Curr. Sci. 78, 391–398.
Singh B. N. 2001 Patterns of inversion polymorphism in three species of the Drosophila melanogaster species group. Ind. J. Exp. Biol. 39, 611–622.
Singh B. N. 2008 Chromosome inversions and linkage disequilibrium in Drosophila Curr. Science 94, 459–464.
Singh B. N. 2010 Drosophila ananassae : a good model species for genetical, behavioural and evolutionary studies. Ind. J. Exp. Biol. 48, 333–345.
Singh B. N. 2013 Genetic polymorphisms in Drosophila. Curr. Sci. 105, 461–469.
Singh B. N. 2018 The Dobzhansky’s concept of genetic coadaptation: Drosophila ananassae is an exception to this concept. J. Genet. 97, 1039–1046.
Singh B. N. 2019 Hundred years of research on inversion polymorphism in Drosophila. Curr. Sci. 117, 761–775.
Singh B. N. and Banerjee R. 1996 Spontaneous recombination in males of Drosophila bipectinata. J. Biosci. 21, 775–779.
Singh B. N. and Mohanty S. 1989 Incomplete penetrance, segregation ratio and crossing-over in Drosophila ananassae. Genet. Iberica 41, 67–82.
Singh B. N. and Mohanty S. 1990 Lack of correlation between crossing-over and chromosome distance between inversions in Drosophila ananassae. Genome 33, 592–595.
Singh B. N. and Mohanty S. 1991 Intra- and interchromosomal effects of heterozygous inversions on crossing-over in the third chromosome of Drosophila ananassae. Ind. J. Exp. Biol. 29, 23–27.
Singh B. N. and Mohanty S. 1992 A spontaneous genetic mosaic in Drosophila ananassae. Curr. Sci. 62, 372–374.
Singh B. N. and Singh A. K. 1987a Intrachromosomal effect of inversion on crossing-over in Drosophila ananassae. Braz. J. Genet. 10, 1–12.
Singh B. N. and Singh A. K. 1987b The effects of heterozygous inversions on crossing over in Drosophila ananassae. Genome 29, 802–805.
Singh A. K. and Singh B. N. 1988a Heterozygous inversions and spontaneous male crossing-over in Drosophila ananassae. Genome 30, 445–450.
Singh B. N. and Singh A. K. 1988b Crossing-over between linked inversions in Drosophila ananassae. Hereditas 109, 15–19.
Singh A. K. and Singh B. N. 1990a Recombination in Drosophila males. Ind. Rev. Life. Sci. 10, 27–53.
Singh B. N. and Singh A. K. 1990b Linkage disequilibrium in laboratory strains of Drosophila ananassae is due to drift. Hereditas 112, 203–208.
Singh B. N. and Yadav J. P. 2015 Status of research on Drosophila ananassae at global level. J. Genet. 94, 785–792.
Sturtevant A. H. 1926 A crossover reducer in Drosophila melanogaster due to inversion of a section of the third chromosome. Biol. Zentrablat. 46, 697–702.
Swift H. 1969 Nuclear physiology and differentiation: a general summary. Genetics 61, 442–461.
Tobari Y. N. and Moriwaki D. 1983 Positive correlation between male recombination and minute mutation frequencies in Drosophila ananassae. Jap. J. Genet. 58, 159–163.
Whittinghill M. 1937 Induced crossing-over in Drosophila males and its probable nature. Genetics 22, 114–129.
Whittinghill M. 1947 Spermatogonial crossing-over between the third chromosomes in the presence of Curly inversion of Drosophila melanogaster. Genetics 32, 608–614.
Acknowledgements
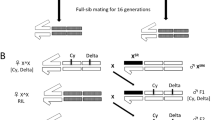
The work of author cited in this review has been supported by the UGC, CSIR and Centre of Advanced Study in Zoology, Banaras Hindu University. Author thank the Current Science Association for granting the permission to reproduce figure 1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: N. G. Prasad
Rights and permissions
About this article
Cite this article
Singh, B.N. Drosophila ananassae: a species characterized by spontaneous male recombination in appreciable frequency. J Genet 99, 12 (2020). https://doi.org/10.1007/s12041-019-1169-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12041-019-1169-z