Abstract
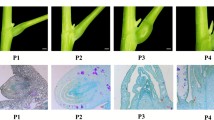
Somatic embryos (SE) of habanero pepper (Capsicum chinense Jacq.) represent persistent deformations in the shoot apical meristem (SAM), which inhibits their capacity to form organs and subsequently plants. In dicotyledonous plants, SAM is formed in the apex, between cotyledons and it plays a central role in postembryonic shoot organ formation. Based on the previous knowledge on the role of some families of gene in the formation, organization and maintenance of the SAM, the expression patterns of WUS, WOX2, NAM, STM, PIN1 and PIN7 genes were analysed, which would allow us to elucidate the possible implication of these genes in SAM deformations in the SE of C. chinense. The results show that the expression patterns of STM and PIN1 in the SE were completely opposite to the respective expression pattern obtained in zygotic embryos (ZE). Moreover, NAM and PIN7 showed an over accumulation of transcripts in SE, compared with ZE. This is the first time in the genus Capsicum that alterations in the expression pattern of key genes of the SE development are reported, as well as its possible implication in the persistent deformations of the SAM.





Similar content being viewed by others
References
Aviles-Viñas S. A., Lecona-Guzmán C. A., Canto-Flick A., López-Erosa S. and Santana-Buzzy N. 2013 Morpho-histological and ultrastructural study on direct somatic embryogenesis of Capsicum chinense Jacq. in liquid medium. Plant Biotechnol. Rep. 7, 277–286.
Belmonte M., Elhiti M., Ashihara H and Stasolla C 2010 Brassinolide- improved development of Brassica napus microspore-derived embryos is associated with increased activities of purine and pyrimidine salvage pathways. Planta 233, 95–107.
Bowman J. and Eshed K. 2000 Formation and maintenance of the shoot apical meristem. Trends Plant Sci. 5, 34–45.
Breuninger H., Rikirsch E., Hermann M., Ueda M. and Laux T. 2008 Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev. Cell 14, 867–876.
Chomczynski P. and Sacchi N. 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159.
Elhiti M., Wally O. S. D., Belmonte M. F., Chan A., Cao Y., Xiang D. et al. 2013 Gene expression analysis in microdissected shoot meristems of Brassica napus microspore-derived embryos with altered SHOOTMERISTEMLESS levels. Planta 237, 1065–1082.
Friml J., Vieten A., Sauer M., Weijers D., Schwarz H., Hamann T. et al. 2003 Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426, 147–153.
Gallavotti A. 2013 The role of auxin in shaping shoot architecture. J. Expt. Bot. 64, 2593–2608.
Gambino G., Minuto M., Bocacci P., Perrone I., Vallania R and Gribaudo I. 2011 Characterization of expression dynamics of WOX homeodomain transcription factors during somatic embryogenesis in Vitis vinifera. J. Expt. Bot. 62, 1089–1101.
Gegas V. C. and Doonan J. H 2006 Expression of cell cycle genes in shoot apical meristems. Plant Mol. Biol. 60, 947–961.
Haecker A., Grob-Hardt R., Geiges B., Sarkar A., Breuninger H., Herrmann M et al. 2004 Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131, 657–668.
Hamant O. and Pautot V. 2010 Plant development: A TALE story. Comptes. Rendus. Biologies. 333, 371–381.
Hay A. and Tsiantis M. 2010 KNOX genes: versatile regulators of plant development and diversity. Development 137, 3153–3165.
Heidmann I., Lange B., Lambalk J., Angenent G. C. and Boutilier K. 2011 Efficient sweet pepper transformation mediated by the BABY BOOM transcription factor. Plant Cell Rep. 30, 1107–1115.
Jenik P. D., Gillmor C. S. and Lukowitz W. 2007 Embryonic patterning in Arabidopsis thaliana. Annu. Rev. Cell Dev. Biol. 23, 207–236.
Kadokura S., Sugimoto K., Tarr P., Suzuki T. and Matsunaga S. 2018 Characterization of somatic embryogenesis initiated from the Arabidopsis shoot apex. Dev. Biol. 442, 13–27.
Livak K. J and Schmittgen T. D 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method Methods 25, 402–408.
Long J. A. and Barton M. K. 1998 The development of apical embryonic pattern in Arabidopsis. Development 125, 3027–3035.
Möller B. and Weijers D. 2009 Auxin control of embryo patterning. Cold Spring Harb–Perspect Biol. 1 a001545.
Murashige T. and Skoog F. 1962 A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plantarum 15, 473–497.
Palovaara J., Hallberg H., Stasolla C. and Hakman I. 2010 Comparative expression patterns analysis of WUSCHEL-related homeobox 2 (WOX2) and WOX8/9 in developing seeds and somatic embryos of the gymnosperm Picea abies. New Phytol. 188, 122–135.
Santana-Buzzy N., Canto-Flick A., Barahona-Pérez F., Montalvo-Peniche M. C., Zapata-Castillo P. Y., Solís-Ruiz A. et al. 2005 Regeneration of habanero pepper (Capsicum chinense Jacq.) via organogenesis. Hort. Sci. 40, 1829–1831.
Souer E., van Houwelingen A., Kloos D, Mol J. and Koes R. 1996 The NO APICAL MERISTEM gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 85, 159–170.
Spinelli S. V., Martin A. P., Viola I. L., Gonzalez D. H. and Palatnik J. F. 2011 A Mechanistic Link between STM and CUC1 during Arabidopsis Development. Plant Physiol. 156, 1894–1904.
Steinitz B., Küsek M., Tabib Y., Paran I. and Zelcer A. 2003 Pepper (C. annuum L.) regenerants obtained by direct somatic embryogenesis fail to develop a shoot. In Vitro Cell Dev-Pl. 39, 296–303.
Su Y. H., Zhao H. Y., Liu Y. B., Zhang C. L., O’Neil S. D. and Zhang X. S 2009 Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. The Plant J, 59, 448–460.
Szakonyi D. and Byrne M. E. 2011 Ribosomal protein L27a is required for growth and patterning in Arabidopsis thaliana. Plant J. 65, 269–281.
Tamura K., Stecher G., Peterson D., Filipski A. and Kumar S. 2013 MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729.
Takada S. and Tasaka M. 2002 Embryonic shoot apical meristem formation in higher plants. J. Plant Res. 115, 411–417.
Thorpe T. A. 2000 Somatic embryogenesis: morphogenesis, physiology, biochemistry and molecular biology. Korean J. Plant Tiss. Cult. 27, 245–258.
Untergasser A., Nijveen H., Rao X., Bisseling Y., Geurts R. and Leunissen J. A. 2007 Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 35, W71–W74.
Van der Graaff E., Laux T. and Rensing S. 2009 The WUS homeobox-containing (WOX) protein family. Genome Biol. 10, 248.
Vanneste S. and Friml J. 2009 Auxin: a trigger for change in plant development. Cell 136, 1005–1016.
Wan H., Yuan W., Ruan M., Ye Q, Wang R, Li Z. et al. 2011 Identification of reference genes for reverse transcription quantitative real-time PCR normalization in pepper (Capsicum annuum L.). Biochem. Bioph. Res. Co. 416, 24–30.
Weijers D., Sauer M., Meurette O., Friml J, Ljung K, Sandberg G et al. 2005 Maintenance of embryonic auxin distribution for apical-basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell 17, 2517–2526.
Zhang Z. and Laux T. 2011 The asymmetric division of the Arabidopsis zygote: from cell polarity to an embryo axis. Sex Plant Reprod. 24, 161–169.
Zazímalová E., Krecek P., Skupa P., Hoyerova K. and Petrásek J. 2007 Polar transport of the plant hormone auxin the role of PIN-FORMED (PIN) proteins. Cell Mol. Life Sci. 64, 1621–1637.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding Editor: H. A. Ranganath
Rights and permissions
About this article
Cite this article
Regla-Márquez, C.F., Avilés-Viñas, S.A., Canto-Flick, A. et al. Genes involved in the deformations of the shoot apical meristem in somatic embryos of Capsicum chinense Jacq.. J Genet 98, 70 (2019). https://doi.org/10.1007/s12041-019-1117-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12041-019-1117-y