Abstract
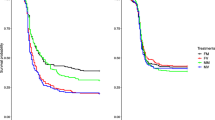
Mating has been widely reported to be a costly event for females. Studies indicate that female cost of mating in terms of fecundity and survivorship can be affected by their mates, leading to antagonistic coevolution between the sexes. However, as of now, there is no evidence that the female cost of mating in terms of immune defence is affected by their mates. We assess the effect of different sized males on antibacterial immune defence and reproductive fitness of their mates. We used a large outbred population of Drososphila melanogaster as the host and Serratia marcescens as the pathogen. We generated three different male phenotypes: small, medium and large, by manipulating larval densities. Compared to females mating with small males, those mating with large males had higher bacterial loads and lower fecundity. There was no significant effect of male phenotype on the fraction of females mated or copulation duration (an indicator of ejaculate investment). Thus, our study is the first clear demonstration that male phenotype can affect the cost of mating to females in terms of their antibacterial immune defence. Mating with large males imposes an additional cost of mating to females in terms of reduced immune defence. The observed results are very likely due to qualitative/quantitative differences in the ejaculates of the three different types of males. If the phenotypic variation that we observed in males in our study is mirrored by genetic variation, then, it can potentially lead to antagonistic coevolution of the sexes over immune defence.
Similar content being viewed by others
References
Atkinson W. D. 1979 A field investigation of larval competition in domestic Drosophila. J. Anim. Ecol. 48, 91–102.
Bretman A., Fricke C. and Chapman T. 2009 Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc. R. Soc. London, Ser. B 276, 1705–1711.
Brown W. D., Bjork A., Schneider K. and Pitnick S. 2004 No evidence that polyandry benefits females in Drosophila melanogaster. Evolution 58, 1242–1250.
Chapman T., Liddle L. F., Kalb J. M., Wolfner M. F. and Partridge L. 1995 Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244.
Chen P. S. 1984 The functional morphology and biochemistry of insect male accessory glands and their secretions. Annu. Rev. Entomol. 29, 233–255.
Chen P. S., Stumm-Zollinger E., Aigaki T., Balmer J., Bienz M. and Bohlen P. 1988 A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell 54, 291–298.
Chippindale A. K. and Rice W. R. 2001 Y chromosome polymorphism is a strong determinant of male fitness in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 98, 5677–5682.
Cox C. R. and Gilmore M. S. 2007 Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infect. Immun. 75, 1565–1576.
Fedorka K. M., Zuk M. and Mousseau T. A. 2004 Immune suppression and the cost of reproduction in the ground cricket, Allonemobius socius. Evolution 58, 2478–2485.
Fedorka K. M., Linder J. E., Winterhalter W. and Promislow D. 2007 Post-mating disparity between potential and realized immune response in Drosophila melanogaster. Proc. R. Soc. London, Ser. B 274, 1211–1217.
Fellowes M. D. E., Kraaijeveld A. and Godfray H. C. J. 1999 The relative fitness of Drosophila melanogaster (Diptera, Drosophilidae) that have successfully defended themselves against the parasitoid Asobara tabida (Hymenoptera, Braconidae). J. Evol. Biol. 12, 123–128.
Flyg C. and Xanthopoulos K. 1983 Insect pathogenic properties of Serratia marcescens. Passive and active resistance to insect immunity studied with protease-deficient and phage-resistant mutants. J. Gen. Microbiol. 129, 453–464.
Flyg C., Kenne K. and Boman H. G. 1980 Insect pathogenic properties of Serratia marcescens: phage-resistant mutants with a decreased resistance to Cecropia immunity and a decreased virulence to Drosophila. J. Gen. Microbiol. 120, 173–181.
Fowler K. and Partridge L. 1989 A cost of mating in female fruitflies. Nature 338, 760–761.
Friberg U. 2006 Male perception of female mating status: its effect on copulation duration, sperm defence and female fitness. Anim. Behav. 72, 1259–1268.
Friberg U. and Arnqvist G. 2003 Fitness effects of female choice: preferred males are detrimental for Drosophila melanogaster females. J. Evol. Biol. 16, 797–811.
Gilchrist A. S. and Partridge L. 2000 Why it is difficult to model sperm displacement in Drosophila melanogaster: the relation between sperm transfer and copulation duration. Evolution 54, 534–542.
Heifetz Y., Lung O., Frongillo E. A. and Wolfner M. F. 2000 The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr. Biol. 10, 99–102.
Herndon L. A. and Wolfner M. F. 1995 A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc. Natl. Acad. Sci. USA 92, 10114–10118.
Holland B. and Rice W. R. 1998 Perspective: chase-away sexual selection: antagonistic seduction versus resistance. Evolution 52, 1–7.
Holland B. and Rice W. R. 1999 Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl. Acad. Sci. USA 96, 5083–5088.
Imroze K. and Prasad N. G. 2011 Sex-specific effect of bacterial infection on components of adult fitness in Drosophila melanogaster. J. Evol. Biol. Res. 3, 79–86.
Kalb J. M., DiBenedetto A. J. and Wolfner M. F. 1993 Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proc. Natl. Acad. Sci. USA 90, 8093–8097.
Kemp D. J. and Rutowski R. L. 2004 A survival cost to mating in a polyandrous butterfly, Colias eurytheme. Oikos 105, 65–70.
Kraaijeveld A. R. and Godfray H. C. 1997 Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature 389, 278–280.
Kuijper B., Stewart A. D. and Rice W. R. 2006 The cost of mating rises non-linearly with copulation frequency in a laboratory population of Drosophila melanogaster. J. Evol. Biol. 19, 1795–1802.
Lawniczak M. K., Barnes A. I., Linklater J. R., Boone J. M., Wigby S. and Chapman T. 2007 Mating and immunity in invertebrates. Trends Ecol. Evol. 22, 48–55.
Lazzaro B. P., Sceurman B. K. and Clark A. G. 2004 Genetic basis of natural variation in D. melanogaster antibacterial immunity. Science 303, 1873–1876.
Lazzaro B. P., Sackton T. B. and Clark A. G. 2006 Genetic variation in Drosophila melanogaster resistance to infection: a comparison across bacteria. Genetics 174, 1539–1554.
Lew T. A., Morrow E. H. and Rice W. R. 2006 Standing genetic variance for female resistance to harm from males and its relationship to intra-locus sexual conflict. Evolution 60, 97–105.
Linder J. E. and Rice W. R. 2005 Natural selection and genetic variation for female resistance to harm from males. J. Evol. Biol. 18, 568–575.
McKean K. A. and Nunney L. 2001 Increased sexual activity reduces male immune function in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 98, 7904–7909.
McKean K. A., Yourth C. P., Lazzaro B. P. and Clark A. G. 2008 The evolutionary costs of immunological maintenance and deployment. BMC Evol. Biol. 8, 76–95.
Moret Y. and Schmid-Hempel P. 2000 Survival for immunity: the price of immune system activation for bumblebee workers. Science 290, 1166–1168.
Nunney L. 1990 Drosophila on oranges: colonization, competition and coexistence. Ecology 71, 1904–1915.
Pitnick S. 1991 Male size influences mate fecundity and remating interval in Drosophila melanogaster. Anim. Behav. 41, 735–745.
Pitnick S. and Garcia-Gonzalez F. 2002 Harm to females increases with male body size in Drosophila melanogaster. Proc. R. Soc. London, Ser. B 269, 1821–1828.
Rice W. R. 1996 Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381, 232–234.
Rolff J. and Siva-Jothy M. T. 2002 Copulation corrupts immunity: a mechanism for a cost of mating in insects. Proc. Natl. Acad. Sci. USA 99, 9916–9918.
Schwarzenbach G. A., Hosken D. J. and Ward P. I. 2005 Sex and immunity in the yellow dung fly Scathophaga stercoraria. J. Evol. Biol. 18, 455–463.
Sheldon B. C. and Verhulst S. 1996 Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321.
Shoemaker K. L., Parsons N. M. and Adamo S. A. 2006 Mating enhances parasite resistance in the cricket Gryllus texensis. Anim. Behav. 71, 371–380.
Short S. M. and Lazzaro B. P. 2010 Female and male genetic contributions to post-mating immune defence in female Drosophila melanogaster. Proc. Biol. Sci. 277, 3649–3657.
Siva-Jothy M. T., Tsubaki Y. and Hooper R. E. 1998 Decreased immune response as a proximate cost of copulation and oviposition in a damselfly. Physiol. Entomol. 23, 274–277.
Swanson W. J., Clark A. G., Waldrip-Dail H. M., Wolfner M. F. and Aquadro C. F. 2001 Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc. Natl. Acad. Sci. USA 98, 7375–7379.
Thomas R. H. 1993 Ecology of body size in Drosophila buzzatii: untangling the effects of temperature and nutrition. Ecol. Entomol. 18, 84–90.
Wigby S. and Chapman T. 2004 Female resistance to male harm evolves in response to manipulation of sexual conflict. Evolution 58, 1028–1037.
Wigby S. and Chapman T. 2005 Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15, 316–321.
Wilkinson G. 1987 Equilibrium analysis of sexual selection in Drosophila melanogaster. Evolution 41, 11–21.
Yanagi S. and Miyatake T. 2003 Costs of mating and egg production in female Callosobruchus chinensis. J. Insect. Physiol. 49, 823–827.
Ye Y. H., Chenoweth S. F. and McGraw E. A. 2009 Effective but costly, evolved mechanisms of defense against a virulent opportunistic pathogen in Drosophila melanogaster. PLoS Pathog. 5, e1000385.
Author information
Authors and Affiliations
Corresponding author
Additional information
Imroze K. and Prasad N. G. 2011 Mating with large males decreases the immune defence of females in Drosophila melanogaster. J. Genet. 90, xx–xx
Rights and permissions
About this article
Cite this article
IMROZE, K., PRASAD, N.G. Mating with large males decreases the immune defence of females in Drosophila melanogaster . J Genet 90, 427–434 (2011). https://doi.org/10.1007/s12041-011-0105-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-011-0105-7