Abstract
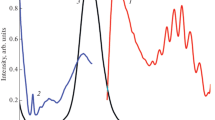
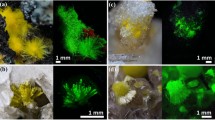
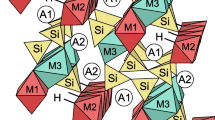
The structural and spectroscopic characteristics of phosphatic ferruginous shale samples from the Bijawar Group rocks from Sagar District of Madhya Pradesh (India) have been probed for identification of uranium species. Fluorapatite (\(\hbox {Ca}_{5}\hbox {(PO}_{4})_{3}\hbox {F}\), FAP) and haematite (\(\upalpha \)-\(\hbox {Fe}_{2}\hbox {O}_{3}\)) were identified as the main phases in the separated mineral concentrates. The photoluminescence (PL) and X-ray absorption near edge spectroscopy (XANES) studies pointed to a strong experimental evidence of both U(IV) and U(VI) oxidation states in the mineral concentrate portion obtained from the same parent host rock. The PL spectrum has confirmed the charge transfer (f–d) transition bands in UV and near-UV regions with emission peaks at ca. 290, 313, 336, 399 and 416 nm, which has been attributed to the substitution of \(\hbox {Ca}^{2+}\) ions by U(IV) in FAP and broad structureless emission due to stabilisation of U(VI) as \(\hbox {UO}_{6}^{6-}\) in haematite. Time-resolved spectroscopy studies have revealed biexponential decay components lasting 2–5 ns for U(IV) species and \(10\,\upmu \hbox {s}\) for U(VI) species. These characterisations revealed the fundamental information about the oxidation state and form of uranium in this region. Remediation measures for the Bijawar region are also suggested.








Similar content being viewed by others
References
Albedri M B, Arar H A and Hameed W O 2014 Determination of natural radioactivity levels in surface soils of old phosphatic mines at Russaifa of Jordan; Int. J. Phys. Res. 4 31–38.
Azami M, Mozafari S M and Rabiee M 2011 Synthesis and solubility of calcium fluoride/hydroxy-fluorapatite nanocrystals for dental applications; Ceram. Int. 37 2007–2014.
Banerjee D M, Khan M W Y, Shrivastav N and Saigal G C 1982 Precambrian phosphorites in the Bijawar rocks of Hirapur-Bassia areas, Sagar district Madhyapradesh; India Miner. Deposita 17 349–362.
Baumann N, Arnold T and Lonschinski M 2012 TRFLS study on speciation of uranium in seepage water and pore water of heavy metal contaminated soil; J. Radioanal. Nucl. 291 673–679.
Beevers C A and McIntyre D B 1946 The atomic structure of fluorapatite and its relation to that of tooth and bone material; Miner. Mag. 27 254–257.
Betancur A F, Pérez F R, Correa M, Del M and Barrero C A 2012 Quantitative approach in iron oxides and oxyhydroxides by vibrational analysis; Opt. Pura. Appl. 45(3) 269–275.
Blasse G 1975 Influence of local charge compensation on site occupation and luminescence of apatites; J. Solid State Chem. 14(2) 181–184.
Burns P C, Miller M L and Ewing R C 1996 \(\text{ U }^{6+}\) minerals and inorganic phases: A comparison and hierarchy of crystal structures; Can. Miner. 34 845–880.
Carnell W T, Liu G K, Williams C W and Reid M F 1991 Analysis of the crystal-field spectra of the actinide tetrafluorides \({\rm UF}_{4}\), \({\rm NpF}_{4}\), and \({\rm PuF}_{4}\); J. Chem. Phys. 95 7194–7203.
Catalano J G, Heald S M, Jachara J M and Brown G E Jr 2004 Spectroscopic and diffraction studies of Uranium speciation in contaminated Vandose Zone sediments from the Hanford Site, Washington State; Environ. Sci. Technol. 38 2822–2828.
Cevik U, Baltas H, Tabak A and Damla N 2010 Radiological and chemical assessment of phosphate rocks in some countries; J. Hazard. Mater. 182 531–535.
Chung C W, Chun J, Wang G and Um W 2014 Effect of iron oxides on the rheological properties of cementitious slurry; Colloids Surf. A 453 94–100.
Cockbain A G and Smith G V 1967 Alkaline-earth-rare-earth silicate and germanate apatites; Mineral. Mag. 36 411–421.
Comodi P, Liu Y and Frezzotti M L 2001 Structural and vibrational behaviour of Fluoroapatite with pressure: Part II. In-situ micro Raman spectroscopic investigation; Phys. Chem. Miner. 28 225–231.
Dar S A, Khan K F, Khan S A, Mir A R, Wani H and Balaram V 2014a Uranium (U) concentration and its genetic significance in the phosphorites of the Paleoproterozoic Bijawar Group of the Lalitpur district, Uttar Pradesh, India; Arab J. Geosci. 7 2237–2248.
Dar S A, Khan K F, Khan Saif A, Khan S and Alam M M 2014b Petromineralogical studies of the paleoproterozoic Phosphorites in the Sonrai basin, Lalitpur District, Uttarpradesh, India; Nat. Resour. Res. 24 339–348.
Dodge C J, Francis A J, Gillow J B, Halada G P, Eng C and Clayton C R 2002 Association of uranium with iron oxides typically formed on corroding steel surfaces; Environ. Sci. Technol. 36 3504–3511.
Duff M C, Morris D E, Hunter D B and Bertsch P M 2000 Spectroscopic characterization of uranium in evaporation basin sediments; Geochim. Cosmochim. Acta 64 1535–1550.
Duff M, Coughlin J and Hunter D 2002 Uranium co-precipitation with iron oxide minerals; Geochim. Cosmochim. Acta 66 3533–3547.
Dzombak D A and Morel F M M 1990 Surface complexation modeling – Hydrous ferric oxide; 24, John Wiley & Sons, New York.
Elliott J C 1994 Structure and chemistry of the apatites and other calcium orthophosphates; Elsevier, New York.
Elliott J C, Wilson R M and Dowker S E P 2002 ‘Apatite Structures’, JCPDS-International Centre for diffraction data; Adv. X-ray Anal. 45 172–181.
Elton E S, Pacheko J S, Bargar J R, Shi Z, Liu J, Kovarik L, Engelhard M H and Felmy A R 2012 Reduction of U(VI) incorporated in the structure of haematite; Environ. Sci. Technol. 46(17) 9428–9436.
Finch R and Murakami T 1999 Systematics and paragenesis of uranium minerals; In: Uranium: Mineralogy, geochemistry and the environment (eds) Burns P C and Finch R, Mineral. Soc. Am. 38 91–180.
Fleet M E and Pan Y 1995 Site preference of rare earth elements in fluorapatite; Am. Mineral. 80 329–335.
Gaft M, Panczer G, Reisfeld R and Uspensky E 2001 Laser-induced time-resolved luminescence as a tool for rare-earth element identification in mineral; Phys. Chem. Mineral. 28 347–363.
Godbole S V, Page A G, Sangeeta Sabharwal S C, Gesland J Y and Sastry M D 2001 UV luminescence of \(\text{ U }^{4+}\) ions in LiYF\(_{4}\) single crystal: Observation of \(\text{5f }^{1}\text{6d }^{1}\)–\(\text{5f }^{2}\) transition; J. Lumin. 93 213.
Görller-Walrand C, Gos M P and D’Olieslager W 1993 The luminescence spectra of \({\rm UF}_{4}\) and \({\rm UCl}_{4}\); Radiochim. Acta 62 55–60.
Gunawardance R P, Howie R A and Glasser F P 1982 Structure of the oxyapatite \({\rm NaY}_{9}({\rm SiO}_{6}){\rm O}_{2}\); Acta Crystllogr. B 38 1564–1566.
Guzman E T R, Rios M S, Garcia J L I and Regil E O 1995 Uranium in phosphate rock and derivatives; J. Radioanal. Nucl. 189(2) 301–306.
Hanesch M 2009 Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies; Geophys. J. Int. 177 941–948.
Hashem E, Swinburne A N, Schulzke C, Evans R C, Platts J A, Kerridge A, Natrajan L S and Baker R J 2013 Emission spectroscopy of uranium (IV) compounds: A combined synthetic, spectroscopic and computational study; RSC Adv. 3 4350–4361.
Hsi C K D and Langmuir D 1985 Adsorption of uranyl on to ferric oxyhydroxides: Application of the surface complexation site-binding model; Geochim. Cosmochim. Acta 49 1931–1941.
Hughes J M, Cameron M and Mariano A N 1991 Rare-earth-element ordering and structural variations in natural rare-earth-bearing apatites; Am. Mineral. 76 1165–1173.
Hughson M R and SenGupta J G 1964 A thorian intermediate member of the britholite-apatite series; Am. Mineral. 49 937.
Jha S K, Shrivastav J P and Bhairam C L 2012 Clay mineralogical studies in the bijawars of the Sonrai Basin: Paleoenvironmental implications and interferences on the uranium mineralization; J. Geol. Soc. India 79 117–134.
Jones D L, Andrewa M B, Swinburne N, Botchway S W, Ward Andrew D and Lloyd J R 2015 Fluorescence spectroscopy and microscopy as tools for monitoring redox transformation of uranium in biological system; Chem. Sci. 6 5133–5138.
Jorge Villar S E, Edwards H G M and Worland M R 2005 Comparative evaluation of Raman spectroscopy at different wavelengths for extremophile exemplars; Orig. Life Evol. Biospheres 35 489–506.
Kay M I, Young R A and Posner A S 1964 Crystal structure of hydroxyapatite; Nature 204 1050–1052.
Kirishima A, Kimura T, Nagaishi R and Tochiyama O 2004 Luminescence properties of tetravalent uranium in aqueous solution; Radiochim. Acta 92 705–710.
Kirm M, Krupa J S and Makhov V N 2003 6d5f and \({\rm 5f}^{2}\) configurations of \({\rm U}_{4}\) doped into \({\rm LiYF}_{4}\) and \({\rm YF}_{3}\) crystal; J. Lumin. 104 85–92.
Krepelova A, Brendler V, Sachs S, Baumann N and Bernhard G 2007 U(VI)– Kaolinite surface complexation in absence and presence of humic acid studies by TRFLS; Environ. Sci. Technol. 41(17) 6142–6147.
Luo Y, Hughes J M, Rakovan J and Pan Y 2009 Site preference of U, F and Sr apatites; Am. Mineral. 94 345–351.
Mackie P E, Elliott J C and Young R A 1972 Monoclinic structure of synthetic Ca,(PO.),CI, chlorapatite; Acta Crystallogr. B 28 1840–1848.
Malek C K, Krupa J C and Guillamont R 1984 Spectra of \({\rm U}^{4+}\) ion at a site of D-2d symmetry; Inorg. Chim. Acta 94 92–95.
Massey M S, Lezama-Pacheco J S, Michel F M and Fendorf S 2014 Uranium incorporation into aluminum-substituted ferrihydrite during iron (II)-induced transformation; Environ. Sci. Process. Impact. 16 2137–2144.
Menzel R G 1968 Uranium, thorium and radium content in phosphate rocks and their possible radiation Hazard;J. Agric. Food. Chem. 16(2) 231–234.
Morris D E, Allen P G, Berg J M, Chisolm-Brause C J, Conradson S D, Donhoe R J, Hess N J, Musgrave J A, Drew Tait C and Nancy J 1996 Speciation of uranium in Fernald soils by molecular spectroscopic methods: Characterization of untreated soils; Environ. Sci. Technol. 30 2322–2331.
Murad E and Schwertmann U 1986 Influence of Al substitution and crystal size on the room temperature Mossabeur spectra of haematite; Clay Clay. Miner. 34 1–6.
Nary Szabo S 1930 The structure of apatite; Z. Kristallogr. 75 387–398.
Panczer G, Gaft M, Reufeld R, Shaval S, Boulan G and Chapagown B 1998 Luminiscence of uranium in natural apatites; J. Alloys Compd. 275–277 269–272.
Pauling L and Henricks S B 1925 The crystal structure of hematite and corundum; J. Am. Chem. Soc. 47(3) 781–790.
Philip G, Fister H and Pauly H 1979 Occupational dose from natural radionuclides in phosphate fertilizers; Radiat. Environ. Biophys. 16 143–156.
Regensperg S, Margot-Roguier C, Froidevaux P, Steinmann P, Junier P and Bernier-Latmani R 2010 Speciation of naturally-accumulated uranium in an organic–rich soil of an alpine region (Switzerland); Geochim. Cosmochim. Acta 74 2082–2098.
Reitz T, Merroun M L, Rossberg A, Steudner R and Solenska-Pobell S 2011 Bioaccumulation of U(VI) by Sulfolobus acidocaldarius under moderate acidic conditions 99; Radiochim. Acta 9 543–554.
Roy M, Bagchi A K, Babu E V S S K, Mishra B and Krishnamurthy P 2004 Petromineragraphy and mineral chemistry of Bituminous Shale hosted uranium mineralization at Sonrai, Lalitpur district, Uttar Pradesh; J. Geol. Soc. India 63 291–298.
Sahu S K, Ajmal P Y, Bhangare R C, Tiwari M and Pandit G G 2014 Natural radioactivity assessment of a phosphate fertilizer plant area; J. Radiat. Res. Appl. Sci. 7 123–128.
Satten R A, Schreiber C L and Wong E Y 1965 Energy levels of \({\rm U}^{4+}\) in an octahedral crystalline field; J. Chem. Phys. 42 162–171.
Shannon R D 1976 Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides; Acta. Crystallogr. A 32 751–761.
Stoch A, Jastrzebski W, Brozek A, Trybalska B, Cichocinska M and Szarawar E 1997 FTIR monitoring of the growth of the carbonate containing apatite layers from simulated and natural body fluids; J. Mol. Struct. 511 287–294.
Tanner P A, Pei Z W, Jun L, Yulong L and Qiang S 1997 Luminiscence of uranium doped strotium borate (\({\rm SrB}_{4}0_{7}\)); J. Phys. Chem. Solids. 58(7) 1143–1146.
Waite T D, Davis J A, Payne T E, Waychunas G A and Xu N 1994 Uranium (VI) adsorption to ferrihydrite: Application of a surface complexation model; Geochim. Cosmochim. Acta 58(24) 5465–5478.
Weigel F 1986 Uranium; In: The chemistry of the actinide elements; 2nd edn, Vol. 1 (eds) Katz J J et al., Chapman and Hall, Springer, Netherlands, Chap. 5, pp. 169–442.
White T J and Zhili D 2003 Structural deviation and crystal chemistry of apatites; Acta. Crystallogr. B 59 1–16.
Willis B T M and Rooksby H P 1952 Crystal structure and anti-ferromagnetism in Haematite; Phys. Soc. London B 65 950–962.
Wright A O, Seltzer M D, Gruber J B and Chai B H T 1995 Site-selective spectroscopy and determination of energy levels in \({\rm Eu}^{3+}\)-doped strontium fluorophosphate; J. Appl. Phys. 78(4) 2456–2467.
Acknowledgements
The authors deeply acknowledge Ms. Parasmani Rajput and Mr S N Jha, RRCAT, Indore for their help in XANES measurement and Mr Raghvendra Reddy, IUC, Indore for his help in Mössbauer measurements. The authors also like to thank Mr A K Rai, the Director, AMD, for granting the permission to pursue this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: N V Chalapathi Rao
Supplementary material pertaining to this article is available on the Journal of Earth System Science website (http://www.ias.ac.in/Journals/Journal_of_Earth_System_Science).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pandit, P., Kumar, S., Bangotra, P. et al. Structural and luminescent characterisation of uraniferous fluorapatite and haematite associated with phosphatic rocks of the Bijawar group in Sagar District, Madhya Pradesh (India). J Earth Syst Sci 127, 110 (2018). https://doi.org/10.1007/s12040-018-1009-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12040-018-1009-1