Abstract
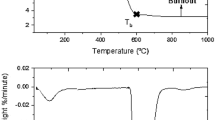
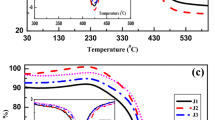
In Part 1 of the present investigation, 37 representative Eocene coal samples of Meghalaya, India were analyzed and their physico-chemical characteristics and the major oxides and minerals present in ash samples were studied for assessing the genesis of these coals. Various statistical tools were also applied to study their genesis. The datasets from Part 1 used in this investigation (Part 2) show the contribution of major oxides towards ash fusion temperatures (AFTs). The regression analysis of high temperature ash (HTA) composition and initial deformation temperature (IDT) show a definite increasing or decreasing trend, which has been used to determine the predictive indices for slagging, fouling, and abrasion propensities during combustion practices. The increase or decrease of IDT is influenced by the increase of Fe2O3, Al2O3, SiO2, and CaO, respectively. Detrital-authigenic index (DAI) calculated from the ash composition and its relation with AFT indicates Sialoferric nature of these coals. The correlation analysis, Principal Component Analysis (PCA), and Hierarchical Cluster Analysis (HCA) were used to study the possible fouling, slagging, and abrasion potentials in boilers during the coal combustion processes. A positive relationship between slagging and heating values of the coal has been found in this study.





Similar content being viewed by others
References
Akar G and Itekoglu U 2007 Relationship between ash fusion temperature (AFT) and coal mineral matter in some Turkish coal ashes; Asian J. Chem. 21 2105–2109.
Benson S A, Hurley J P, Zygarlicke C J, Steadman E N, and Erickson T 1993 Predicting ash behavior in utility boilers; Energy Fuels 7 746–754.
Barnes I 2009 Slagging and fouling in coal-fired boilers; CCC/147, 43p, ISBN 978-92-9029-466-5 (www.ieacoal.org.uk).
Baruah B P 2008 Environmental studies around Makum coalfield, Margherita (Thesis); Dibrugarh University, Assam, India; 118p.
Baruah B P 2009 Environmental studies around Makum coalfield, Assam, India; Lambert Academic Publishing; ISBN 978-3-8383-2322-0, pp. 16–18.
Chatfield C and Collin A J 1980 Introduction to multivariate analysis; Chapman and Hall, London & New York; ISBN: 0-412-16030-7.
Dutta Roy R K 1940 A critical study of some Indian coal ashes; The Twelfth Ordinary General Meeting of National Institute of Sciences of India, Kolkatta 6(3) 539–548.
European Commission Directorate-General XIII Telecommunications 1994 The role of fuel additives to control environment emissions and ash fouling, Final report Information Market and Exploitation of Research L-2920 Luxembourg; 4.
Fryda L, Sobrino C, Cieplik M, and Van de Kamp W L 2010 Study on ash deposition under oxyfuel combustion of coal/biomass blends; Fuel 89 (8) 1889–1902.
Gupta S K, Wall T F, Creelman R A, and Gupta R P 1998 Ash fusion temperatures and the transformations of coal ash particles to slag; Fuel Processing Technology 56 33–43.
Huffman G P, Huggins F E, and Dunmyre G R 1981 Investigation of the high temperature behavior of coal ash in reducing and oxidizing atmospheres; Fuel 60 585–597.
Heinzel T, Siegle V, Spliethoff H, and Hein K R G 1998 Investigation of slagging in pulverized fuel co-combustion of biomass and coal at a pilot-scale test facility; Fuel Processing Technology 54 (1–3) 109–125.
IS: 1355. 1959 Methods of test for ash of coal and coke.
IS: 12891. 1990 Indian Standard Method of determination of fusibility of ash of coal, coke and lignite.
Kazagic A and Smajevic I 2007 Experimental investigation of ash behaviour and emissions during combustion of Bosnian coal and biomass; Energy 32 (10) 2006–2016.
Lloyd W G, Riley J T, Zhou S, Risen M A, and Tibbits R L 1993 Ash fusion temperatures under oxidizing conditions; Energy Fuels 7 490–494.
Li H X, Ninomiya Y, Dong Z B, and Zhang M X 2006 Applications of the factsage to predict the ash melting behaviour in reducing conditions; Chinese J. Chem. Eng. 14 (6) 784–789.
Mishra H K and Ghosh R K 1996 Geology, petrology and utilisation potential of some tertiary coals of northeastern region of India; Int. J. Coal Geol. 30 65–100.
Munir S and Nimmo W 2010 Potential slagging and fouling problems associated with biomass-coal blends in coal-fired boilers; J. Pakistan Inst. Chem. Eng. 38 1–11.
Masia A A T, Buhre B J P, Gupta R P, and Wall T F 2007 Characterizing ash of biomass and waste; Fuel Processing Technology 88 (11–12) 1071–1081.
Nayak B 2013 Mineral matter and the nature of pyrite in some high sulphur tertiary coals of Meghalaya, northeast India; J. Geol. Soc. India 81 203–214.
Pronobis M 2005 Evaluation of the influence of biomass co-combustion on boiler furnace slagging by means of fusibility correlations; Biomass and Bioenergy 28 (4) 375–383.
Reifenstein A P 1999 Behaviour of selected minerals in an improved ash fusion test: Quartz, potassium feldspar, sodium feldspar, kaolinite, illite, calcite, dolomite, siderite, pyrite and apatite; Fuel 78 1449–1461.
Sharma A, Saikia B K, and Baruah B P 2012 Maceral contents of tertiary Indian coals and their relationship with calorific values; Int. J. Inn. Res. Develop. 1 (7) 196–203.
Singh M P and Singh A K 2000 Petrographic characteristics and depositional conditions of Eocene coals of platform basins, Meghalaya, India; Int. J. Coal Geol. 42 (4) 315–356.
Singh P K, Singh A L, Kumar A, and Singh M P 2011 A study on removal of selected major elements from Indonesian coal through bacteria: Environmental implications; Proceedings, International Conference on Energy, Environment, Sustainable Development, Bangkok, Thailand 75 925–935.
Singh P K, Singh A L, Kumar A, and Singh M P 2012a Mixed bacterial consortium as an emerging tool to remove hazardous trace metals from coal; Fuel 102 227–230.
Singh P K, Singh M P, Singh A K, and Naik A S 2012b Petrographic and geochemical characterization of coals from Tiru valley, Nagaland, NE India; Energy, Exploration and Exploitation 30 (2) 171–192.
Singh A K, Singh M P, and Singh P K 2013 Petrological investigations of Oligocene coals from foreland basin of northeast India; Energy, Exploration and Exploitation 31 (6) 909–936.
Skorupska N M 1993 Coal specifications impact on power station performance; IEACR/52, IEA Coal Research London UK, 120p.
Song W J, Tang L H, Zhu X D, Wu Y Q, Rong Y Q and Zhu Z B et al. 2009 Fusibility and flow properties of coal ash and slag; Fuel 88 297–304.
Song W J, Tang L H, Zhu X D, Wu Y Q, Zhu Z B, and Koyama S 2010 Effect of coal ash composition on ash fusion temperature; Energy Fuels 24 182– 189.
Van Dyk J C, Melzer S, and Sobiecki A 2006 Mineral matter transformation during Sasol-Lurgi fixed bed dry bottom gasification–utilization of HT-XRD and FactSage modeling; Minerals Eng. 19 1126–1135.
Vassilev S V, Kitano K, Takeda S, and Tsurue T 1995 Influence of mineral and chemical composition of coal ashes on their fusibility; Fuel Processing Technology 45 (1) 27–51.
Vassileva C G and Vassilev S V 2002 Relations between ash-fusion temperatures and chemical and mineral composition of some Bulgarian Coals; Bulgarian Academy of Sciences, pp. 61–66.
Vassilev S V and Vassileva C G 2007 A new approach for the classification of coal fly ashes based on their origin, composition, properties and behaviour; Fuel 86 1490–1512.
Vassilev S and Vessileva C 2009 A new approach for the combined chemical and mineral classification of the inorganic in the coal. 1. Chemical and mineral classification system; Fuel 88 235–245.
Vamvuka D and Zografos D 2004 Predicting the behaviour of ash from agricultural wastes during combustion; Fuel 83 (14–15) 2051–2057.
Ward C R 2002 Analysis and significance of mineral matter in coal seams; Int. J. Coal Geol. 50 135–168.
Wynteri C I, May L, Oliver F W, Hall J A, Hoffman E J, Kumar A and Christopher L 2004 Correlation of coal calorific value and sulphur content with57Fe Mössbauer spectral absorption; Hyperfine Interactions 153 147–152.
Yang J G, Deng F R, Zhao H, and Cen K F 2006 Mineral conversion of coal ash in fusing process and the influence to ash-fusion points; Proc. CSEE (Chinese edn) 26 122–126.
Acknowledgements
The authors are thankful to the Ministry of Steel, Govt. of India, New Delhi and CSIR, New Delhi for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, A., Saikia, A., Khare, P. et al. The chemical composition of tertiary Indian coal ash and its combustion behaviour – a statistical approach: Part 2. J Earth Syst Sci 123, 1439–1449 (2014). https://doi.org/10.1007/s12040-014-0475-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12040-014-0475-3