Abstract
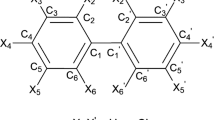
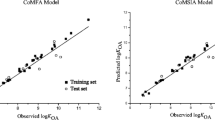
The quantitative structure-property relationship (QSPR) technique is used to gauge the n-octanol/water partition coefficient (log KOW) and enthalpy of vaporization (∆vapHm) of 133 Polychlorinated Biphenyls (PCBs) using conceptual density functional theory (CDFT)-based global reactivity and information-theory (IT) based parameters. Regression models are established using linear and multi-linear relationships to correlate the observed physicochemical properties of PCBs with the predicted ones. The study explored the significance of CDFT and IT descriptors, and based on the calculation of Pearson correlation coefficient values, the selection of suitable descriptors is made for successful QSPR models of selected PCBs. It is found that some of the CDFT parameters are highly correlated with the IT parameters, as suggested by their high Pearson correlation coefficient values for PCB systems. The regression model generated using the descriptors IG, g1, g2, EA, η for predicting log KOW and IF, g3, η, SS, SGBP for predicting ∆vapHm gives R2 value of 0.9342 and 0.8662, respectively, for the selected 133 PCB congeners. Furthermore, to verify the descriptor selection, a machine learning approach is also used to develop QSPR models in this study.
Graphical Abstract
QSPR modelling using CDFT and information theory-based descriptors for predicting n-octanol/water partition coefficient and enthalpy of vaporization for the selected PCBs









Similar content being viewed by others
References
Hansch C, Maloney P, Fujita T and Muir R 1962 Correlation of biological activity of phenoxyacetic acids with Hammett substituent constants and partition coefficients Nature 194 178
Hansch C and Fujita T 1964 p-σ-π Analysis. A method for the correlation of biological activity and chemical structure J. Am. Chem. Soc. 86 1616
Zhao Y H, Cronin M T and Dearden J C 1998 Quantitative Structure-Activity Relationships of Chemicals Acting by Non-polar Narcosis— Theoretical Considerations Quant. Struct.-Act. Relat. 17 131
Roy K, Kar S and Das R N 2015 Understanding the basics of QSAR for applications in pharmaceutical sciences and risk assessment 1st edn. (Ed.) (Oxford: Academic Press)
Russom C L, Bradbury S P, Broderius S J, Hammermeister D E and Drummond R A 1997 Predicting modes of toxic action from chemical structure: Acute toxicity in the fathead minnow (Pimephales promelas) Environ. Toxicol. Chem. 16 948
Khadikar P V, Mather K C, Singh S, Phadnis A, Shrivastava A and Mandaloi M 2002 Study on quantitative structure–toxicity relationships of benzene derivatives acting by narcosis Bioorg. Med. Chem. 10 1761
Karelson M, Lobanov V S and Katritzky A R 1996 Quantum-chemical descriptors in QSAR/QSPR studies Chem. Rev. 96 1027
Kubinyi H 1993 QSAR: Hansch Analysis and Related Approaches (Weinheim: Wiley-VCH) p.240
Zhao Y H, Ji G D, Cronin M T D and Dearden J C 1998 QSAR study of the toxicity of benzoic acids to Vibrio fischeri, Daphnia magna and carp Sci. Total Environ. 216 205
Raevsky O and Skvortsov V 2005 Quantifying hydrogen bonding in QSAR and molecular modeling SAR QSAR Environ. Res. 16 287
Kim K H 1993 3D-quantitative structure-activity relationships: describing hydrophobic interactions directly from 3D structures using a comparative molecular field analysis (CoMFA) approach Quant. Struct. -Act Relat. 12 232
Sabet R and Fassihi A 2008 QSAR study of antimicrobial 3-hydroxypyridine-4-one and 3-hydroxypyran-4-one derivatives using different chemometric tools Int. J. Mol. Sci. 9 2407
Livingstone D J and Manallack D T 2003 Neural networks in 3D QSAR QSAR Comb. Sci. 22 510
Lin Z H, Long H X, Bo Z, Wang Y Q and Wu Y Z 2008 New descriptors of amino acids and their application to peptide QSAR study Peptides 29 1798
Puri S, Chickos J S and Welsh W J 2003 Three-dimensional quantitative structure-property relationship (3D-QSPR) models for prediction of thermodynamic properties of polychlorinated biphenyls (PCBs): enthalpies of fusion and their application to estimates of enthalpies of sublimation and aqueous solubilities J. Chem. Inf. Comput. Sci. 43 55
Padmanabhan J, Parthasarathi R, Subramanian V and Chattaraj P K 2007 Using QSPR models to predict the enthalpy of vaporization of 209 polychlorinated biphenyl congeners QSAR Comb. Sci. 26 227
Giri S, Roy D R, Van Damme S, Bultinck P, Subramanian V and Chattaraj P K 2008 An atom counting QSPR protocol QSAR Comb. Sci. 27 208
Lü W, Chen Y, Liu M, Chen X and Hu Z 2007 QSPR prediction of n-octanol/water partition coefficient for polychlorinated biphenyls Chemosphere 69 469
Masand V H, El-Sayed N N, Bambole M U, Patil V R and Thakur S D 2019 Multiple quantitative structure-activity relationships (QSARs) analysis for orally active trypanocidal N-myristoyltransferase inhibitors J. Mol. Struct. 1175 481
Katritzky A R, Slavov S H, Dobchev D A and Karelson M 2008 QSAR modeling of the antifungal activity against Candida albicans for a diverse set of organic compounds Bioorg. Med. Chem. 16 7055
He W, Yan F, Jia Q, Xia S and Wang Q 2018 QSAR models for describing the toxicological effects of ILs against Staphylococcus aureus based on norm indexes Chemosphere 195 831
Deokar H S, Puranik P and Kulkarni V M 2009 QSAR analysis of N-myristoyltransferase inhibitors: antifungal activity of benzofurans Med. Chem. Res. 18 206
Wang L, Ding J, Pan L, Cao D, Jiang H and Ding X 2021 Quantum chemical descriptors in quantitative structure–activity relationship models and their applications Chemometr. Intell. Lab. Syst. 217 104384
Chhajed M, Shrivastava A K, Chhajed A, Taile V, Prachand S and Jain S 2017 Computational evaluation of 2-amino-5-sulphonamido-1, 3, 4-thiadiazoles as human carbonic anhydrase-IX inhibitors: an insight into the structural requirement for the anticancer activity against HEK 293 Med. Chem. Res. 26 2272
RamaKrishna K, Rao C and Rao R S 2015 Chemoinformatics Part I: molecular descriptors in omnimetrics research J. Appl. Chem. (Lumami, India) 4 1024
Ivanciuc O 2013 Chemical graphs, molecular matrices and topological indices in chemoinformatics and quantitative structure-activity relationships Curr. Comput. Aided Drug Des. 9 153
Roy P P, Paul S, Mitra I and Roy K 2009 On two novel parameters for validation of predictive QSAR models Molecules 14 1660
Gramatica P 2013 On the Development and Validation of QSAR Models Reisfeld B and Mayeno A (Eds.) In Computational Toxicology Methods in Molecular Biology (NJ: Humana Press)
Roy K and Mitra I 2012 On the use of the metric rm 2 as an effective tool for validation of QSAR models in computational drug design and predictive toxicology Mini-Rev. Med. Chem. 12 491
De P, Kar S, Ambure P and Roy K 2022 Prediction reliability of QSAR models: an overview of various validation tools Arch. Toxicol. 96 1279
Guha R and Jurs P C 2004 Development of linear, ensemble, and nonlinear models for the prediction and interpretation of the biological activity of a set of PDGFR inhibitors J. Chem. Inf. Comput. Sci. 44 2179
Hemmateenejad B, Safarpour M A, Miri R and Nesari N 2005 Toward an optimal procedure for PC-ANN model building: prediction of the carcinogenic activity of a large set of drugs J. Chem. Inf. Model. 45 190
Itskowitz P and Tropsha A 2005 k nearest neighbors QSAR modeling as a variational problem: theory and applications J. Chem. Inf. Model. 45 777
Devillers J (Ed.) 1996 Neural Networks in QSAR and Drug Design (Academic Press: London)
Pan S, Gupta A K, Subramanian V and Chattaraj P K 2017 Quantitative structure-activity/Property/Toxicity relationships through conceptual density functional theory-based reactivity descriptors: In Pharmaceutical Sciences: Breakthroughs in Research and Practice (IGI Global) p.1517
Parthasarathi R, Subramanian V, Roy D R and Chattaraj P K 2004 Electrophilicity index as a possible descriptor of biological activity Bioorg. Med. Chem. 12 5533
Parthasarathi R, Padmanabhan J, Subramanian V, Maiti B and Chattaraj P K 2004 Toxicity analysis of 33′ 44′ 5-pentachloro biphenyl through chemical reactivity and selectivity profiles Curr. Sci. 86 535
Roy D R, Parthasarathi R, Subramanian V and Chattaraj P K 2006 An electrophilicity based analysis of toxicity of aromatic compounds towards Tetrahymena pyriformis QSAR Comb. Sci. 25 114
Padmanabhan J, Parthasarathi R, Subramanian V and Chattaraj P K 2006 Group philicity and electrophilicity as possible descriptors for modeling ecotoxicity applied to chlorophenols Chem. Res. Toxicol. 19 356
Pan S, Gupta A, Roy D, Sharma R, Subramanian V, Mitra A and Chattaraj P K 2016 Application of conceptual density functional theory in developing qsar models and their usefulness in the prediction of biological activity and toxicity of molecules (New York: Apple Academic Press) p. 183
Jana G, Pal R, Sural S and Chattaraj P K 2020 Quantitative Structure-Toxicity Relationship Models Based on Hydrophobicity and Electrophilicity, In Ecotoxicological QSARs (New York: Humana) p.661
Pal R, Pal G, Jana G and Chattaraj P K 2019 An In Silico QSAR Model Study Using Electrophilicity as a Possible Descriptor Against T. Brucei Int. J. Chemoinform. Chem. Eng. 8 57
Pal R, Jana G, Sural S and Chattaraj P K 2019 Hydrophobicity versus electrophilicity: A new protocol toward quantitative structure–toxicity relationship Chem. Biol. Drug Des. 93 1083
Jana G, Pal R, Sural S and Chattaraj P K 2020 Quantitative structure-toxicity relationship: An “in silico study” using electrophilicity and hydrophobicity as descriptors Int. J. Quantum Chem. 120 e26097
Pauling L 1960 The Nature of the Chemical Bond 3rd edn. (Ed.) (New York: Cornell Univ. Press)
Sen K D and Jorgenson C K 1987 Electronegativity. Structure and Bonding (Springer: Berlin)
Parr R G, Donnelly R A, Levy M and Palke W E 1978 Electronegativity: the density functional viewpoint J. Chem. Phys. 68 3801
Parr R G, Szentpály L V and Liu S 1999 Electrophilicity index J. Am. Chem. Soc. 121 1922
Chattaraj P K, Sarkar U and Roy D R 2006 Electrophilicity index Chem. Rev. 106 2065
Chattaraj P K and Parr R G 1993 Density Functional Theory of Chemical Hardness, Sen K D (Ed.) In Chemical Hardness, Structure and Bonding (Berlin: Springer) p. 11
Pearson R G 1997 Chemical Hardness: Applications from Molecules to Solids (Wiley VcH: Weinheim)
Yang W and Mortier W J 1986 The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines J. Am. Chem. Soc. 108 5708
Yang W T and Parr R G 1985 Hardness, softness, and the fukui function in the electronic theory of metals and catalysis Proc. Natl. Acad. Sci. U. S. A. 82 6723
Parr R G and Yang W 1989 Density-Functional Theory of Atoms and Molecules (Oxford Univ. Press: New York)
Nalewajski R F and Parr R G 2000 Information theory, atoms in molecules, and molecular similarity Proc. Natl. Acad. Sci. 97 8879
Nalewajski R F and Parr R G 2001 Information theory thermodynamics of molecules and their Hirshfeld fragments J. Phys. Chem. A 105 7391
Nalewajski R F, Witka E and Michalak A 2002 Information distance analysis of molecular electron densities Int. J. Quant. Chem. 87 198
Nalewajski R F 2003 Information principles in the theory of electronic structure Chem. Phys. Lett. 372 28
Ayers P W 2006 Information Theory, the Shape Function, and the Hirshfeld Atom Theor. Chem. Acc. 115 370
Borgoo A, Geerlings P and Sen K D 2008 Electron density and Fisher information of Dirac-Fock atoms Phys. Lett. A 372 5106
Geerlings P and Borgoo A 2011 Information carriers and (reading them through) information theory in quantum chemistry Phys. Chem. Chem. Phys. 13 911
Alipour M 2013 Wave vector, local momentum and local coordinate from the perspective of information theory Mol. Phys. 111 3246
Alipour M 2015 Making a happy match between orbital-free density functional theory and information energy density Chem. Phys. Lett. 635 210
Xu J H, Guo L Y, Su H F, Gao X, Wu X F, Wang W G, et al. 2017 Heptanuclear CoII5CoIII2 Cluster as Efficient Water Oxidation Catalyst Inorg. Chem. 56 1591
Chen J, Liu S, Li M, Rong C and Liu S 2020 A density functional theory and information-theoretic approach study of chiral molecules in external electric fields Chem. Phys. Lett. 757 137858
Cao X, Rong C, Zhong A, Lu T and Liu S 2018 Molecular acidity: An accurate description with information-theoretic approach in density functional reactivity theory J. Comput. Chem. 39 117
Rong C, Wang B, Zhao D and Liu S 2020 Information-theoretic approach in density functional theory and its recent applications to chemical problems Wiley Interdiscip. Rev. Comput. Mol. Sci. 10 e1461
He X, Li M, Yu D, Wang B, Zhao D, Rong C and Liu S 2021 Conformational changes for porphyrinoid derivatives: an information-theoretic approach study Theor. Chem. Acc. 140 1
Cao X, Liu S, Rong C, Lu T and Liu S 2017 Is there a generalized anomeric effect? Analyses from energy components and information-theoretic quantities from density functional reactivity theory Chem. Phys. Lett. 687 131
Liu S B 2009 Conceptual Density Functional Theory and Some Recent Developments Acta Phys. Chim. Sin. 25 590
Chattaraj P K, Chamorro E and Fuentealba P 1999 Chemical bonding and reactivity: a local thermodynamic viewpoint Chem. Phys. Lett. 314 114
Shannon C E 1948 A mathematical theory of communication Bell Syst. Tech. J. 27 379
Ghosh S K, Berkowitz M and Parr R G 1984 Transcription of ground-state density-functional theory into a local thermodynamics Proc. Natl. Acad. Sci. 81 8028
Fisher R A 1925 Theory of statistical estimation Math. Proc. Cambridge Philos. Soc. 22 700
Onicescu O 1966 Theorie de l’information energie informationelle C. R. Acad. Sci. A-B 263 841
Liu S B 2016 Information-theoretic approach in density functional reactivity theory Acta Phys.-Chim. Sin. 32 98
Liu S 2019 Identity for Kullback-Leibler divergence in density functional reactivity theory J. Chem. Phys. 151 141103
Kullback S and Leibler R A 1951 On information and sufficiency Ann. Math. Stat. 22 79
Rényi A 1970 Probability Theory (North-Holland: Amsterdam)
Liu S B, Rong C Y, Wu Z M and Lu T 2015 Rényi entropy, Tsallis entropy and Onicescu information energy in density functional reactivity theory Acta Phys.-Chim. Sin. 31 2057
Nagy Á and Romera E 2015 Relative Rényi entropy and fidelity susceptibility Europhys. Lett. 109 60002
Poddar A, Pal R, Rong C and Chattaraj P K 2023 A conceptual DFT and information-theoretic approach towards QSPR modeling in polychlorobiphenyls J. Math. Chem. 61 1143
Chen Y, Cai X, Jiang L and Li Y 2016 Prediction of octanol-air partition coefficients for polychlorinated biphenyls (PCBs) using 3D-QSAR models Ecotoxicol. Environ. Saf. 124 202
Padmanabhan J, Parthasarathi R, Subramanian V and Chattaraj P K 2006 QSPR models for polychlorinated biphenyls: n-Octanol/water partition coefficient Bioorg. Med. Chem. 14 1021
Falconer R L and Bidleman T F 1994 Vapor pressures and predicted particle/gas distributions of polychlorinated biphenyl congeners as functions of temperature and ortho-chlorine substitution Atmos. Environ. 28 547
Puri S, Chickos J S and Welsh W J 2001 Determination of vaporization enthalpies of polychlorinated biphenyls by correlation gas chromatography Anal. Chem. 73 1480
Puri S, Chickos J S and Welsh W J 2003 Three-dimensional quantitative structure− property relationship (3D-QSPR) models for prediction of thermodynamic properties of polychlorinated biphenyls (PCBs): enthalpies of fusion and their application to estimates of enthalpies of sublimation and aqueous solubilities J. Chem. Inf. Comp. Sci. 43 55
Polishchuk P G, Muratov E N, Artemenko A G, Kolumbin O G, Muratov N N, and Kuz’min V E 2009 Application of random forest approach to QSAR prediction of aquatic toxicity J. Chem. Inf. Model. 49 2481
Emrarian M, Sohrabi M R, Goudarzi N and Tadayon F 2020 Quantitative structure-property relationship (QSPR) study to predict retention time of polycyclic aromatic hydrocarbons using the random forest and artificial neural network methods Struct. Chem. 31 1281
Kovdienko N A, Polishchuk P G, Muratov E N, Artemenko A G, Kuz’min V E, Gorb L, et al. 2010 Application of random forest and multiple linear regression techniques to QSPR prediction of an aqueous solubility for military compounds Mol. Inform. 29 394
Becke A D 1993 Density-Functional Thermochemistry. III. The Role of Exact Exchange J. Chem. Phys. 98 5648
Lee C, Yang W and Parr R G 1988 Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density Phys. Rev. B 37 785
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, et al. 2016 Gaussian 16 (Gaussian Inc: Wallingford, UK)
Lu T and Chen F 2012 Multiwfn: A multifunctional wavefunction analyzer J. Comput. Chem. 33 580
Van Rossum G and Drake F L 2009 Introduction to Python 3: python documentation manual part 1 Scotts Valley, CA: CreateSpace
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al., 2011 Scikit-learn: Machine Learning in Python J. Mach. Learn. Res. 12 2825
Hunter J D 2007 Matplotlib: A 2D graphics environment Comput. Sci. Eng. 9 90
Acknowledgements
PKC would like to thank DST, New Delhi, for the J. C. Bose National Fellowship, grant number SR/S2/JCB-09/2009. AP thanks IIT Kharagpur for her fellowships. AC also thanks IIT Kharagpur. The authors gratefully acknowledge the high-performance supercomputing system of IIT Kharagpur, the “PARAM Shakti.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this article, financial and/or otherwise.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poddar, A., Chordia, A. & Chattaraj, P.K. QSPR models for n-octanol/water partition coefficient and enthalpy of vaporization using CDFT and information theory-based descriptors. J Chem Sci 136, 23 (2024). https://doi.org/10.1007/s12039-024-02250-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-024-02250-0