Abstract
The conformational state and noncovalent interaction of protonated dopamine (p-dopamine) play an important role in its key and lock binding with its receptors. Hence, understanding of the role of weak noncovalent interactions in the stability of the higher order structures of the p-dopamine is desired. In this study, we have combined the spectroscopic and quantum chemical calculation studies to understand the role of noncovalent interactions in the stability of the dimers and trimers of p-dopamine in the aqueous medium. The intensity of the UV–Visible spectra of p-dopamine increases and shows a red shift with increasing concentrations suggesting the presence of the higher order structures of p-dopamine in the aqueous medium. The quantum chemical calculations and AIM studies of the different structures of its dimer and trimers suggest the presence of N–H3+…π, C–H…π, π…π weak interactions along with conventional N–H…O hydrogen bond. The calculated peak positions of the UV–Visible spectra of different clusters show that the higher order of clusters show red shifted peak position compared to the monomer and the red shifted peak is more evident in the clusters having noncovalent interactions.
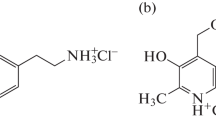
Graphical abstract
Weak noncovalent interactions stabilise higher order clusters of protonated dopamine leading to red shift in the UV-visible spectra as shown by quantum chemical calculations.







Similar content being viewed by others
References
Korshunov K S, Blakemore L J and Trombley P Q 2017 Dopamine: a modulator of circadian rhythms in the central nervous system Front. Cell. Neurosci. 11 91
Jaber M, Robinson S W, Missale C and Caron M G 1996 Dopamine receptors and brain function Neuropharmacology 35 1503
Lot T Y 1993 Mechanisms of action of dopamine in the peripheral nervous system of chicks and rats J. Pharm. Pharmacol. 45 896
Rubí B and Maechler P 2010 Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let’s seek the balance Endocrinology 151 5570
Murty V P, Tompary A, Adcock R A and Davachi L 2017 Selectivity in postencoding connectivity with high-level visual cortex is associated with reward-motivated memory J. Neurosci. 37 537
Alcaro A, Huber R and Panksepp J 2007 Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective Brain Res. Rev. 56 283
Marsden C A 2006 Dopamine: the rewarding years Br. J. Pharmacol. 147(Suppl 1) S136
Thorp J, Clewett D and Riegel M 2020 Two routes to incidental memory under arousal: dopamine and norepinephrine J. Neurosci. 40 1790
Burbulla L F, Song P, Mazzulli J R, Zampese E, Wong Y C, Jeon S, et al. 2017 Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease Science 357 1255
Surmeier D J, Obeso J A and Halliday G M 2017 Selective neuronal vulnerability in Parkinson disease Nat. Rev. Neurosci. 18 101
Meisenzahl E M, Schmitt G J, Scheuerecker J and Möller H J 2007 The role of dopamine for the pathophysiology of schizophrenia Int. Rev. Psychiatry 19 337
Cabezas C, Peña I, López J C and Alonso J L 2013 Seven conformers of neutral dopamine revealed in the gas phase J. Phys. Chem. Lett. 4 486
Urban J J, Cronin C W, Roberts R R and Famini G R 1997 Conformational preferences of 2-phenethylamines: A computational study of substituent and solvent effects on the intramolecular amine−aryl interactions in charged and neutral 2-phenethylamines J. Am. Chem. Soc. 119 12292
Fausto R, Ribeiro M J S and de Lima J J P 1999 A molecular orbital study on the conformational properties of dopamine [1,2-benzenediol-4(2-aminoethyl)] and dopamine cation J. Mol. Struct. 484 181
Callear S K, Johnston A, McLain S E and Imberti S 2015 Conformation and interactions of dopamine hydrochloride in solution J. Chem. Phys. 142 014502
Bustard T M and Egan R S 1971 The conformation of dopamine hydrochloride Tetrahedron 27 4457
Bergin R and Carlstrom D 1968 The structure of the catecholamines: II: The crystal structure of dopamine hydrochloride Acta Cryst. B 24 1506
Giesecke J 1980 Refinement of the structure of dopamine hydrochloride Acta Cryst. B 36 178
Park S, Lee N S, Lee S J B and o T K C S, 2000 Vibrational analysis of dopamine neutral base based on density functional force field Bull. Korean Chem. Soc. 21 1035
Lagutschenkov A, Langer J, Berden G, Oomens J and Dopfer O 2011 Infrared spectra of protonated neurotransmitters: dopamine Phys. Chem. Chem. Phys. 13 2815
Zhai C, Ma H, Sun F, Li L and Song A 2016 Experimental and theoretical study on the interaction of dopamine hydrochloride with H2O J. Mol. Liq. 215 481
Berfield J L, Wang L C and Reith M E 1999 Which form of dopamine is the substrate for the human dopamine transporter: the cationic or the uncharged species? J. Biol. Chem. 274 4876
Granot J 1976 Nmr studies of catecholamines. Acid dissociation equilibria in aqueous solutions FEBS Lett. 67 271
Solmajer P, Kocjan D and Solmajer T 1983 Conformational study of catecholamines in solution Z. Naturforsch. C 38 758
Alagona G and Ghio C 1996 The effect of intramolecular H-bonds on the aqueous solution continuum description of the N-protonated form of dopamine Chem. Phys. 204 239
Nagy P I, Alagona G and Ghio C 1999 Theoretical studies on the conformation of protonated dopamine in the gas phase and in aqueous solution J. Am. Chem. Soc. 121 4804
de Moraes E E, Tonel M Z, Fagan S B and Barbosa M C 2019 Density functional theory study of π-aromatic interaction of benzene, phenol, catechol, dopamine isolated dimers and adsorbed on graphene surface J. Mol. Model. 25 302
Durdagi S, Salmas R E, Stein M, Yurtsever M and Seeman P 2016 Binding interactions of dopamine and apomorphine in D2high and D2low states of human dopamine D2 receptor using computational and experimental techniques ACS Chem. Neurosci. 7 185
Bueschbell B, Barreto C A V, Preto A J, Schiedel A C and Moreira I S 2019 A complete assessment of dopamine receptor- ligand interactions through computational methods Molecules 24 1196
Floresca C Z and Schetz J A 2004 Dopamine receptor microdomains involved in molecular recognition and the regulation of drug affinity and function J. Recept. Signal Transduct. Res. 24 207
Salmas R E, Yurtsever M, Stein M and Durdagi S 2015 Modeling and protein engineering studies of active and inactive states of human dopamine D2 receptor (D2R) and investigation of drug/receptor interactions Mol. Divers. 19 321
Grimme S 2006 Semiempirical GGA-type density functional constructed with a long-range dispersion correction J. Comput. Chem. 27 1787
Miertuš S, Scrocco E and Tomasi J 1981 Electrostatic interaction of a solute with a continuum. A direct utilizaion of ab initio molecular potentials for the prevision of solvent effects Chem. Phys. 55 117
Dubinets N O, Safonov A A and Bagaturyants A A 2016 Structures and binding energies of the naphthalene dimer in its ground and excited states J. Phys. Chem. A 120 2779
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Petersson G A, Nakatsuji H, Li X, Caricato M, Marenich A V, Bloino J, Janesko B G, Gomperts R, Mennucci B, Hratchian H P, Ortiz J V, Izmaylov A F, Sonnenberg J L, Williams, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski V G, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr J A, Peralta J E, Ogliaro F, Bearpark M J, Heyd J J, Brothers E N, Kudin K N, Staroverov V N, Keith T A, Kobayashi R, Normand J, Raghavachari K, Rendell A P, Burant J C, Iyengar S S, Tomasi J, Cossi M, Millam J M, Klene M, Adamo C, Cammi R, Ochterski J W, Martin R L, Morokuma K, Farkas O, Foresman J B and Fox D J 2016 Gaussian 16 Rev. C.01 Wallingford CT
Bader R F W 1990 Atoms in Molecules: A Quantum Theory (Oxford: Clarendon Press)
AIM2000 2001 J. Comput. Chem. 22 545
Koch U and Popelier P L A 1995 Characterization of C-H-O hydrogen bonds on the basis of the charge density J. Phys. Chem. 99 9747
Popelier P L A 1998 Characterization of a dihydrogen bond on the basis of the electron density J. Phys. Chem. A 102 1873
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chowdhury, A., Singh, P.C. Role of the weak noncovalent interactions in the stability of the aggregated protonated dopamine in the aqueous solution: spectroscopic and quantum chemical calculation studies. J Chem Sci 134, 25 (2022). https://doi.org/10.1007/s12039-021-02014-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-021-02014-0