Abstract
A simple and clean methodology for the preparation of ligand grafted mercaptopropyl silane functionalized copper (0) nanocluster [LGMPS Cu (0)] has been reported. The catalyst was synthesized by the post-modification of mercaptopropyl silica with pyridine moiety and finally anchoring of Cu (0) produced by in situ reductions of copper salt which enhanced the stability to copper (0) nanoparticles. The catalyst was then characterized with FTIR, XRD, TGA, XPS, EDX, SEM, TEM and AAS. Its application has been studied for O-benzylation and O-allylation of phenols and also in one-pot synthesis of β-amino ketones via Mannich reaction. Moreover, the catalyst was recyclable for up to six runs, making it more sustainable. The recycled catalyst was also characterized using FTIR and TEM which confirmed that the functional groups present in the catalyst remained intact but the size of the catalyst increased due to agglomeration of nanoclusters after the sixth cycle of reusability.
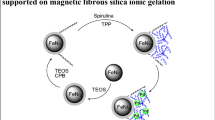
Graphical abstract
The present work deals with the functionalization of copper (0) nanocluster onto mercaptopropyl silica ligated with 2-bromopyridine and its applications for the C–O and C–N bond-forming reactions.
















Similar content being viewed by others
References
(a) Reeves C J, Kasar A K and Menezes P L 2021 Tribological performance of Environment-friendly ionic liquids for high-temperature applications J. Clean. Prod. 279 123666; (b) Chowhan B, Kour J, Gupta M and Paul S 2021 Green synthesis of bis(pyrazol-5-ole) and pyrazolopyranopyrimidine derivatives through mechanochemistry using chitosan as a biodegradable catalyst ChemistrySelect 6 7922
Najeeb J, Naeem S, Nazar M F, Naseem K and Shehzad U 2021 Green chemistry: evolution in architecting schemes for perfecting the synthesis methodology of the functionalized nanomaterials ChemistrySelect 6 3101
(a) Chowhan B, Gupta M and Sharma N 2020 Fabrication and characterization of adenine‐grafted carbon‐modified amorphous ZnO with enhanced catalytic activity Appl. Organomet. Chem. 34 6013; (b) Sharma N, Gupta M, Chowhan B and Frontera A, 2021 Magnetically separable nanocatalyst (IL@ CuFe2O4-L-Tyr-TiO2/TiTCIL): preparation, characterization and its applications in 1, 2, 3-triazole synthesis and in photodegradation of MB J. Mol. Struct. 1224 129029; (c) Baig R N and Varma R S 2013 Magnetically retrievable catalysts for organic synthesis Chem. Commun. 49 752; (d) Chowhan B, Gupta M and Sharma N 2019 designing of ultrafine PdNPs immobilized pyridinic‐N doped carbon and evaluation of its catalytic potential for konevenagel condensation, synthesis of 4H‐pyran derivatives and nitroreduction ChemistrySelect 4 12689; (e) Ferella F 2020 A review on management and recycling of spent selective catalytic reduction catalysts J. Clean. Prod. 246 118990
(a) García-Garrido S E, Francos J, Cadierno V, Basset J M and Polshettiwar V 2011 Chemistry by nanocatalysis: first example of a solid-supported RAPTA complex for organic reactions in aqueous medium ChemSusChem.4 104; (b) Fihri A, Cha D, Bouhrara M, Almana N and Polshettiwar V 2012 Fibrous nano-silica (KCC-1)-supported palladium catalyst: suzuki coupling reactions under sustainable conditions ChemSusChem. 5 85; (c) Madhavan N, Jones C W and Weck M 2008 Rational approach to polymer-supported catalysts: synergy between catalytic reaction mechanism and polymer design Acc. Chem. Res. 41 1153
Boruah J J, Das S P, Ankireddy S R, Gogoi S R and Islam N S 2013 Merrifield resin supported peroxomolybdenum (VI) compounds: recoverable heterogeneous catalysts for the efficient, selective and mild oxidation of organic sulfides with H2O2 Green Chem. 15 2944
(a) Polshettiwar V, Len C and Fihri A 2009 Silica-supported palladium: sustainable catalysts for cross-coupling reactions Coord. Chem. Rev. 253 2599; (b) Polshettiwar V and Molnar A 2007 Silica-supported Pd catalysts for Heck coupling reactions Tetrahedron 30 6949
Cheng T, Zhao Q, Zhang D and Liu G 2015 Transition-metal-functionalized ordered mesoporoussilicas: an overview of sustainable chiral catalysts for enantioselective transformations Green Chem. 17 2100
Borah B J, Dutta D, Saikia P P, Barua N C and Dutta D K 2011 Stabilization of Cu (0)-nanoparticles into the nanopores of modified montmorillonite: an implication on the catalytic approach for “Click” reaction between azides and terminal alkynes Green Chem. 13 3453
(a) Ravikovich A M 1964 Antioxidants for minerals and synthetic lubricating oils Chem. Techno. Fuel Oil. 11 64; (b) Shreve R N and Brink Jr J A 1977 Chemical Process Industries (No. 4th Edition) (Location: McGraw-Hill Book Co.); (c) Lebedev N N 1984 Chemistry and technology of basic organic and petrochemical Synthesis 1; (d) Tareque M H, Ismail M, Chakravarty P, Rana A A and Saha M 2006 Benzylation of phenol with benzyl alcohol in the presence of sulphuric acid Bangladesh J. Sci. Ind. Res. 41 257
(a) Nametkin S S, Baskakov Y A and Melnikov N N 1951 Synthesis of some alkyl and aryl phenoxyacetic acid Zh. Obsh. Khim. 12 2146; (b) Belov P S and Isagulyants V I 1964 Alkylation of phenols with cyclic alcohols in the presence of cation exchange resin Ku-2 Zh. Prikl. Khim. 37 2505
(a) Banerjee S K, Gupta B D and Singh K J 1982 Chem. Soc. Chem. Commun. 815; (b) Lissel M, Schmidt S and Neumann B 1986 Dimethyl carbonate as a methylating agent under phase transfer catalytic conditions Synthesis 1986 382; (c) Srivastava P and Srivastava R 2007 Catalytic investigations of calix [4] arene scaffold-based phase transfer catalyst Tetrahedron Lett. 48 4489; (d) Benaglia M, Cinquini M, Cozzi F and Tocco G 2002 Synthesis of a poly (ethylene glycol)-supported tetrakis ammonium salt: a recyclable phase-transfer catalyst of improved catalytic efficiency Tetrahedron Lett. 43 3391; (e) Albanese D, Benaglia M, Landini D, Maia A, Lupi V and Penso M 2002 Use of a quaternary ammonium salt supported on a liposoluble poly (ethylene glycol) matrix for laboratory and industrial synthetic applications of phase-transfer catalysis Ind. Eng. Chem. Res. 41 4928; (f) Tamami B and Ghasemi S 2008Nucleophilic substitution reactions using polyacrylamide-based phase transfer catalyst in organic and aqueous media J. Iran. Chem. Soc. 5 S26; (g) Chen Z X, Xu G Y, Yang G C and Wang W 2004 Preparation of non-cross-linked polystyrene-supported quaternary ammonium salts and use as phase transfer catalysts under microwave React. Funct. Polym. 61 139; (h) Denmark S E, Weintraub R C and Gould N D 2012 Effects of charge separation, effective concentration, and aggregate formation on the phase transfer catalyzed alkylation of phenol J. Am. Chem. Soc. 134 13415; (i) Coleman M T and LeBlanc G 2010 Use of diethoxymethane as a solvent for phase transfer-catalyzed o-alkylation of phenols Org. Process Res. Dev. 14 732; (j) Badri M, Brunet J J and Perron R 1992 Ionic liquids as solvents for the regioselective O-alkylation of C, O ambident nucleophiles Tetrahedron Lett. 33 4435; (k) Mohanazadeh F and Aghvami M 2007 Ionic liquids as reagent and reaction medium: preparation of alkyl aryl ethers Monatsh. für Chem. 138 47; (l) Lourenço N M and Afonso C A 2003 Ionic liquid as an efficient promoting medium for two-phase nucleophilic displacement reactions Tetrahedron 59 789; (m) Lee J C, Yuk J Y and Cho S H 1995 Facile synthesis of alkyl phenyl ethers using cesium carbonate Synth. Commun. 25 1367; (n) Godfrey Jr J D, Mueller R H, Sedergran T C, Soundararajan N and Colandrea V J 1994 Improved synthesis of aryl 1, 1-dimethylpropargyl ethers Tetrahedron Lett. 35 6405; (o) Taniguchi H and Nomura E 1988 Catalytic activity of an octopus-type calixarene on the formation of ethers Chem. Lett. 171 773; (p) Zhang M, Flynn D L and Hanson P R 2007 Oligomericbenzylsulfonium salts: Facile benzylation via high-load ROMP reagents J. Org. Chem. 72 3194; (q) Thangapriya C, Ilaamirthamani S and Kumarraja M 2020 Highly regioselective O-allylation of phenol derivatives using MMZCu; (I) Y catalyst Synth. Commun. 50 361
Enders D, Moser M, Geibel G and Laufer M C 2004 Diastereo-and enantioselective synthesis of differently N, O-protected 1, 3-amino alcohols with three neighbouring stereogenic centers Synthesis 12 2040
Mukhopadhyay M, Bhatia B and Iqbal J 1997 Cobalt catalyzed multiple component condensation route to β-acetamido carbonyl compound libraries Tetrahedron Lett. 38 1083
Bagheri I, Mohammadi L, Zadsirjan V and Heravi M M 2021 Organocatalyzed asymmetric Mannich reaction: an update ChemistrySelect 6 1008
Hussain M, Liu J, Zhang Z, Hu M, Li Y and Min X 2018 Green synthesis and theoretical study of β-amino esters via PPh3-catalyzed mannich reaction ChemistrySelect 3 8787
Arend M, Westermann B and Risch N 1998 Modern variants of the Mannich reaction Angew Chem. Int. Ed. 37 1044
Kobayashi S and Ueno M 2004 Comprehensive Asymmetric Catalysis (Springer: Berlin)
Kobinata K, Uramoto M, Nishii M, Kusakabe H, Nakamura G and Isono K 1980 Neopolyoxins A, B, and C, new chitin synthetase inhibitors Agric. Biol. Chem. 44 1709
Sharghi H, Razavi S F, Aberi M, Tavakoli F and Shekouhy M 2020 The Co2+ complex of [7-Hydroxy-4-methyl-8-coumarinyl] glycine as a nanocatalyst for the synthesis and biological evaluation of new mannich bases of benzimidazoles and benzothiazoles ChemistrySelect 5 2662
Racane L, Tralic-Kulenovic V and Fiser-Jakic L 2001 Synthesis of bis-substituted amidinobenzothiazoles as potential anti-HIV agents Heterocycles 55 2085
Kashiyama E, Hutchinson I, Chua M S, Stinson S F, Phillips L R, Kaur G, et al. 1999 Antitumor benzothiazoles, 8: synthesis, metabolic formation, and biological properties of the C-and N-oxidation products of antitumor 2-(4-aminophenyl)benzothiazoles J. Med. Chem. 42 4172
Bhusare S R, Pawar R P and Vibhute Y B 2001 Synthesis and antibacterial activity of some new 2-(substituted phenyl sulfonamido)-6-substituted benzothiazoles Indian J. Heterocycl. Chem. 11 79
Boosa V, Varimalla S, Dumpalapally M, Gutta N, Velisoju V K, Nama N and Akula V 2021 Influence of Brønsted acid sites on chemoselective synthesis of pyrrolidones over H-ZSM-5 supported copper catalyst Appl. Catal. B Environ. 292 120177
Baw H 1926 Quart J. Indian Chem. Soc. 3 101
Irlapati N R, Adlington R M, Conte A, Pritchard G J, Marquez R and Baldwin J E 2004 Total synthesis of pyridovericin Tetrahedron 60 9307
Shah S T A, Khan K M, Hussain H, Anwar M U, Fecker M and Voelter W 2005 Cesium fluoride-Celite: a solid base for efficient syntheses of aromatic esters and ethers Tetrahedron 61 6652
Curtin D Y and Wilhelm M 1958 Alkylation of cresol salts: use of azo coupling to establish orientation J. Org. Chem. 23 9
Meyer H and Bernhauer K 1929 About the alkylation of aromatic compounds Monatsch. Chem. 53 721
Gutekunst G O and Gray H L 1922 The 6-alkyloxy quinalbines J. Am. Chem. Soc. 44 1741
Goering H L and Jacobson R R 1958 A kinetic study of the ortho-claisen rearrangement J. Am. Chem. Soc. 80 3277
Tarbell D S and Wilson J W 1942 The rearrangement of 4-crotyloxy-3, 5-dichlorobenzoic acid J. Am. Chem. Soc. 64 1066
Xiao S, He Y, Xu G and Liu Q 2015 Investigation on Claisen rearrangement of allyl phenyl ethers in near-critical water Res. Chem. Intermed. 41 3299
Marcinkiewicz S, Green J and Mamalis P 1961 The relation between the Claisen rearrangement of allyl ethers and their electronic structure: rearrangement of N-allylamines Tetrahedron 14 208
Rao H S P and Senthilkumar S P 2001 A convenient procedure for the synthesis of allyl and benzyl ethers from alcohols and phenols J. Chem. Sci. 113 191
Nand White W and Slater C D 1961 The ortho-claisen rearrangement, V: the products of rearrangement of allyl X-phenyl ethers J. Org. Chem. 26 3631
Shuikin NI, Viktorova E A, Pokrovskaya I E and Malysheva T G 1961 Alkylation of phenols with mixed-functional compounds Russ. Chem. Bull. 10 1547
Yi L, Lei H S, Zou J H and Xu X J 1991 The Mannich reaction between aromatic ketones, aromatic aldehydes and aromatic amines Synthesis 9 717
Wang L M, Han JW, Sheng J, Fan J Y and Tian H 2005 Yb(OTf)3 catalyzed mannich reaction of acetophenone witharomatic aldehydes and aromatic amines: three component one-pot synthesis of β-amino ketone derivatives Chin. J. Org. Chem. 25 591
Kumar V, Sharma U, Verma P K, Kumar N and Singh B 2011 Silica-supported boric acid with ionic liquid: a novel recyclable catalytic system for one-pot three-component Mannich reaction Chem. Pharm. Bull. 59 639
Yi L, Zou JH, Lei HS, Lin X and Zhang M 1991 The Mannich reaction of cyclic ketones, aromatic aldehydes and aromatic amines Org. Prep. Proced. Int. 23 673
Yang D C, Zhang G L, Yang N and Zhong Y G 2000 The Mannich reaction of 4-methylacetophenone with aromatic aldehydes and aromatic amines Chem. J. Chin. Univ. 21 1694
Yi W B and Cai C 2006 Mannich-type reactions of aromatic aldehydes, anilines, and methyl ketones in fluorousbiphase systems created by rare earth (III) perfluorooctanesulfonates catalysts in fluorous media J. Fluor. Chem. 12 71515
Mansoor S S, Aswin K, Logaiya K and Sudhan S P N 2015 An efficient synthesis of β-amino ketone compounds through one-pot three-component Mannich-type reactions using bismuth nitrate as catalyst J. Saudi Chem. Soc. 19 379
Acknowledgements
We are grateful to Director, SAIF, Punjab University, Chandigarh for TEM and XRD and also to the Head, SAIF, IIT Bombay for recording SEM images and also EDAX measurements. We are also thankful to Head, SAIF, IIT Roorkee for thermogravimetric analysis. Bushra Chowhan is also thankful to CSIR, New Delhi (Reference File. No.: 09/100(0199)/ 2017-EMR-1). The authors are also grateful to the Department of Chemistry, University of Jammu, Jammu and the Funding agencies for instruments like NMR (DST-PURSE) and FTIR. We are also thankful to IICT, Hyderabad for the XPS study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gupta, M., Chowhan, B., Gupta, M. et al. Ligand grafted mercaptopropyl silane functionalized copper (0) nanocluster: preparation and applications for C–O and C–N bond-forming reactions. J Chem Sci 134, 16 (2022). https://doi.org/10.1007/s12039-021-02009-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-021-02009-x