Abstract
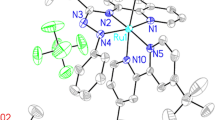
Reaction of [(arene)MCl2]2 with bidentate 4, 5-diazafluorene-9-one (dafo) and derived Schiff-base ligands (L1–L3) in the presence of ammonium hexafluorophosphate yielded mononuclear cationic complexes having general formula [(arene)MLCl]PF6 {M = Ru, arene = benzene (1, 5, 9); M = Ru, arene = p-cymene (4, 8); M = Rh, arene = Cp* (2, 6, 10); M = Ir, arene = Cp* (3, 7, 11); L = 4, 5-diazafluorene-9-one (L1), N-(4, 5-diazafluoren-9-ylidene)aniline (L2), N-(4, 5-diazafluoren-9-ylidene)phenyl hydrazine (L3)}. All these complexes were isolated as hexafluorophosphate salts and characterized by spectral and analytical techniques such as FT-IR, UV-vis, NMR spectroscopy and ESI-Mass spectrometry. Complexes 1-3 were characterized by X-ray crystallographic studies, which indicated NN′ bidentate coordination of the ligands through pyridine nitrogen atoms of the ligand. To evaluate the biological properties of these complexes, antibacterial and antioxidant experiments have been carried out. The complexes 8, 9 and 11 exhibited antibacterial activity against Gram-positive bacteria. Results also show that the compounds possess prominent antioxidant activity against DPPH radicals.
Graphical abstract
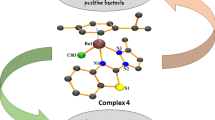
The reaction of [(arene)MCl2]2 with 4, 5- diazafluorene-9-one (dafo) and derived Schiff-base ligands yielded mononuclear cationic complexes having the general formula [(arene)MLCl]PF6. Some of the complexes exhibited antibacterial activity against Gram-positive bacteria. Results also show that the compounds possess prominent antioxidant activity against DPPH radicals.







Similar content being viewed by others
References
Krishnamoorthy M, Hakobyan S, Ramstedt M and Gautrot J E 2014 Surface-initiated polymer brushes in the biomedical field: Applications in membrane science, biosensing, cell culture, regenerative medicine and antibacterial coatings Chem. Rev. 114 10976
Li J, Wang G, Zhu H, Zhang M, Zheng X, Di Z, et al. 2014 Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer Sci. Rep. 4 4359
Zhang H, Liu H B and Yue J M 2014 Organic carbonates from natural sources Chem. Rev. 114 883
Liu R, Chen X, Chakraborty S, Lemke J J, Hayouka Z, Chow C, et al. 2014 Tuning the biological activity profile of antibacterial polymers via subunit substitution pattern J. Am. Chem. Soc. 136 4410
Fasciani C, Silvero M J, Anghel M A, Arguello G A, Becerra M C and Scaiano J C 2014 Aspartame-stabilised gold-silver bimetallic biocompatible nanostructures with plasmonic photothermal properties, antibacterial activity, and long-term stability J. Am. Chem. Soc. 136 17394
Dodbele S, Martinez C D and Troutman J M 2014 Species differences in alternative substrate utilization by the antibacterial target undecaprenyl pyrophosphate synthase Biochem. 53 5042
Levy S B and Marshall B 2004 Antibacterial resistance worldwide: causes, challenges and responses Nat. Med. 10 122
Gold H S and Moellering R C 1996 Antimicrobial-drug resistance N. Engl. J. Med. 335 1445
Sun D, Zhang W, Lv M, Yang E, Zhao Q and Wang W 2015 Antibacterial activity of ruthenium(II) polypyridyl complex manipulated by membrane permeability and cell morphology Bioorg. Med. Chem. Lett. 25 2068
Gichumbi J M, Omondi B, Lazarus G, Singh M, Shaikh N, Chenia H Y and Friedrich H B 2017 Influence of halogen substitution in the ligand sphere on the antitumor and antibacterial activity of half-sandwich ruthenium(II) complexes [RuX(η6-arene)(C5H4N-2-CH=N-Ar)]+ Z. Anorg. Allg. Chem. 643 699
Diengdoh D F 2017 Evaluation of phytochemicals and antioxidant activity in underutilised wild edible plants of Meghalaya state, India Int. J. ChemTech. Res. 10 674
Clarke M J 2002 Ruthenium metallopharmaceuticals Coord. Chem. Rev. 232 69
Mladenova R, Ignatova M, Manolova N, Petrova T and Rashkov I 2002 Preparation, characterization and biological activity of Schiff base compounds derived from 8-hyroxyquinoline-2-carboxaldehyde and Jeffamines ED® Eur. Polym. J. 38 989
Singh K, Barwa M S and Tyagi P 2006 Synthesis, characterization and biological studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with bidentate Schiff bases derived by heterocyclic ketone Eur. J. Med. Chem. 41 147
Panneerselvam P, Nair R R, Vijayalakshmi G, Subramanian E H and Sridhar S K 2005 Synthesis of Schiff bases of 4-(4-aminophenyl)-morpholine as potential antimicrobial agents Eur. J. Med. Chem. 40 225
Abebe A and Hailemariam T 2016 Synthesis and assessment of antibacterial activities of ruthenium (III) mixed ligand complexes containing 1, 10-phenanthroline and guanide Bioinorg. Chem. Appl. 2016 1
Gopinath K, Karthika V, Gowri S, Senthilkumar V, Kumaresan S and Arumugam A 2014 Antibacterial activity of ruthenium nanoparticles synthesized using Gloriosa superba L. leaf extract J. Nanostruct. Chem. 4 83
Lam P L, Lu G L, Hon K M, Lee K W, Ho C L, Wang X, et al. 2014 Development of ruthenium (II) complexes as topical antibiotics against methicillin resistant Staphylococcus aureus Dalton Trans. 43 3949
Annibale V T and Song D T 2016 Coordination chemistry and applications of versatile 4,5-diazafluorene derivatives Dalton Trans. 45 32
(a) Adhikari S, Kaminsky W and Kollipara M R 2017 Investigation of the coordination chemistry of multidentate azine Shiff-base ligands towards d6 half-sandwich metal complexes J. Organomet. Chem. 848 95; (b) Palepu N R, Premkumar J R, Verma A K, Bhattacharjee K, Joshi S R, Forbes S, Mozharivskyj Y and Kollipara M R 2018 Antibacterial, in vitro antitumor activity and structural studies of rhodium and iridium complexes featuring the two positional isomers of pyridine carbaldehyde picolinic hydrazone ligand Arab. J. Chem 11 5 714
Bennett M A, Huang T N, Matheson T W, Smith A K, Ittel S and Nickerson W 1982 (η6-Hexamethylbenzene) Ruthenium complexes Inorg. Synth. 21 74
Dkhar L, Kaminsky W, Poluri K M and Kollipara M R 2019 Versatile coordination modes of benzothiazole hydrazone derivatives towards Ru(II), Rh(III) and Ir(III) complexes and their reactivity studies with azides and activated alkynes J. Organomet. Chem. 891 54
Henderson L J, Fronczek J F R and Cherry W R 1984 Selective perturbation of ligand field excited states in polypyridine ruthenium(II) complexes J. Am. Chem. Soc. 106 5876
Crysalis P R O, release (2012) Version 1.171.36.20. Agilent Technologies, Yarnton
Sheldrick G M 2015 SHELXT-Integrated space-group and crystal-structure determination Acta Cryst. A 71 3
Sheldrick G M 2015 Crystal structure refinement with SHELXL Acta Cryst. C 71 3
Farrugia L J 1997 ORTEP-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI) J. Appl. Crystallogr. 30 565
Blois M S 1958 Antioxidant determinations by the use of a stable free radical Nature 181 1199
Baran M F, Durap F, Aydemir M and Baysal A 2016 Transfer hydrogenation of aryl ketones with homogeneous ruthenium catalysts containing diazafluorene ligands Appl. Organometal. Chem. 30 1030
Prasad K T, Therrien B and Kollipara M R 2008 Cationic half-sandwich complexes (Rh, Ir, Ru) containing 2-substituted-1,8-naphthyridine chelating ligands: Synthesis, X-ray structure analyses and spectroscopic studies J. Organomet. Chem. 693 3049
Adhikari S, Hussain O, Phillips R M and Kollipara M R 2018 Half-sandwich d6 metal complexes comprising of 2-substituted-1,8-napthyridine ligands with unexpected bonding modes: Synthesis, structural and anti-cancer studies J. Organomet. Chem. 854 27
Adhikari S, Sutradhar D, Shepherd S L, Phillips R M, Chandra A K and Kollipara M R 2016 Neutral and cationic half-sandwich arene ruthenium, Cp*Rh and Cp*Ir oximato and oxime complexes: Synthesis, structural, DFT and biological studies J. Organomet. Chem. 820 70
Gupta G, Park S, Lee S S and Kim J 2011 Syntheses and Molecular Structure of Dinuclear Transition Metal Complexes Bridged by Dipyridylamine Derivative Ligands Z. Anorg. Allg. Chem. 637 1516
Tsolis T, Manos M J, Karkabounas S, Zelovitis I and Garoufis A 2014 Synthesis, X-ray structure determination, cytotoxicity and interactions with 9-methylguanine, of ruthenium (II) η6-arene complexes J. Organomet. Chem. 768 1
Govindaswamy P, Canivet J, Therrien B, Süss-Fink G, Stepnicka P and Ludvík J 2007 Mono and dinuclear rhodium, iridium and ruthenium complexes containing chelating 2,2’-bipyrimidine ligands: synthesis, molecular structure, electrochemistry and catalytic properties J. Organomet. Chem. 692 3664
Tweedy B G 1964 Plant extracts with metal ions as potential antimicrobial agents Phytopathology 55 910
Prabhakaran R, Geetha A, Thilagavathi M, Karvembu R, Krishnan V, Bertagnolli H and Natarajan K 2004 Synthesis, characterization, EXAFS investigation and antibacterial activities of new ruthenium (III) complexes containing tetradentate Schiff base J. Inorg. Biochem. 98 2131
Ozsoy N, Candoken E and Akev N 2009 Implications for degenerative disorders: Antioxidative activity, total phenols, flavonoids, ascorbic acid, β-carotene and β-tocopherol in Aloe vera Oxid. Med. Cell. Longev. 2 99
Acknowledgment
Carley Giffert L Nongpiur is grateful to Larica Pathaw for her help in the initial stages of this work. Carley also thanks Professor A. K. Singh, Department of Biochemistry, NEHU, SAIF-CDRI, Lucknow and SAIF-NEHU, Shillong for spectral analyses.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nongpiur, C.G.L., Tripathi, D.K., Poluri, K.M. et al. Ruthenium, rhodium and iridium complexes containing diazafluorene derivative ligands: synthesis and biological studies. J Chem Sci 134, 23 (2022). https://doi.org/10.1007/s12039-021-02004-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-021-02004-2