Abstract
An efficient and inexpensive method for the N-alkylation of pyrimidines using ammonium sulfate coated Hydro-Thermal-Carbone (HTC) (AS@HTC) as reused heterogeneous catalyst was developed. The catalyst was characterized by several analytical techniques such as SEM, XRD, and FTIR. The effect of various parameters was studied including catalyst loading, mole ratio, to achieve excellent selectivity and yields in 80–90%. Significantly, the present protocol offers the use of an inexpensive and environmentally friendly catalyst and simple workup. The simplicity of the procedure, excellent yield of the products, and the recyclability of the catalyst are the main advantages of this method.
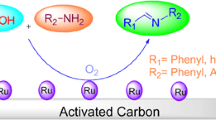
Graphic Abstract
Ammonium sulfate coated Hydro-Thermal-Carbone (HTC) (AS@HTC); an efficient and reused heterogeneous catalyst of the N-alkylation of pyrimidines was developed. Excellent selectivity and yields (80–90%) toward N1-alkylpyrimidines were achieved. Significantly, the present protocol offers the use of an inexpensive and environmentally friendly catalyst and simple workup.



Similar content being viewed by others
References
Salehi P, Dabiri M, Zolfigol M A and Fard M A B 2003 Silica sulfuric acid as an efficient and reusable reagent for crossed-aldol condensation of ketones with aromatic aldehydes under solvent-free conditions Tetrahedron Lett. 44 2889
Moghaddas M, Davoodnia A, Heravi M M and Tavakoli-Hoseini N 2012 Sulfonated Carbon Catalyzed Biginelli Reaction for One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones and -thiones Chin. J. Catal. 33 706
Deshmane C A, Wright M W, Lachgar A, Rohlfing M, Liu Z, Le J and Hanson B E 2013 A comparative study of solid carbon acid catalysts for the esterification of free fatty acids for biodiesel production. Evidence for the leaching of colloidal carbon Biores. Tech. 147 597
Qi X and Liu N Lian Y 2015 Carbonaceous microspheres prepared by hydrothermal carbonization of glucose for direct use in catalytic dehydration of fructose RSC Adv. 5 17526
Liu T, Li Z, Li W, Shi C and Wang Y 2013 Preparation and characterization of biomass carbon-based solid acid catalyst for the esterification of oleic acid with methanol Biores. Tech. 133 618
Climent M J, Corma A, Iborra S and Sabater J M 2014 Heterogeneous Catalysis for Tandem Reactions ACS Catal. 4 870
Fraile J M and Saavedra C J 2017 Application of Heterogeneous Catalysts in the First Steps of the Oseltamivir Synthesis Catalysts 7 393
Akhil V N and Ganapati D Y 2018 Cu2O nanoparticles supported hydrothermal carbon microspheres as catalyst for propargylamine synthesis Mol. Cat. 451 209
Filoklis D P, Maham T, Sam C and Maria-Magdalena T 2014 Esterification of levulinic acid into ethyl levulinate catalysed by sulfonated hydrothermal carbons Chin. J. Catal. 35 929
Doke D S, Umbarkar S B, Gawande M B, Zboril R and Biradar A V 2017 Environmentally Benign Bioderived Carbon Microspheres-Supported Molybdena Nanoparticles as Catalyst for the Epoxidation Reaction Chem. Eng. 5 904
Reddy B M and Patil M K 2009 Organic Syntheses and Transformations Catalyzed by Sulfated Zirconia Chem. Rev. 109 2185
Venkatesh K R, Hu J, Dogan C, Tierney J W and Wender I 1995 Sulfated metal oxides and related solid acids: comparison of protonic acid strengths Energy Fuels 9 888
Drago R S and Kob N 1997 Acidity and reactivity of sulfated zirconia and metal-doped sulfated zirconia J. Phys. Chem. B 101 3360
Niedballa U and Vorbrueggen H 1976 Synthesis of nucleosides 17. A general synthesis of N-glycosides. 6. On the mechanism of the stannic chloride catalyzed silyl Hilbert-Johnson reaction J. Org. Chem. 41 2084
Vorbruggen H, Krolikiewicz K and Bennua B 1981 Nucleoside syntheses, XXII1) Nucleoside synthesis with trimethylsilyl triflate and perchlorate as catalysts Chem. Ber. 114 1234
Vorbruggen H and Ruh-Polenz C 2000 Synthesis of Nucleosides Org. React. 55 1
Chengyuan L, Weihui J, Shunjun D, Han S and Gennian M 2017 Effective Synthesis of Nucleosides Utilizing O-Acetyl-Glycosyl Chlorides as Glycosyl Donors in the Absence of Catalyst: Mechanism Revision and Application to Silyl-Hilbert-Johnson Reaction Molecules 22 84
Bennua-Skalmowski B, Krolikiewicz K and Vorbruggen H 1995 A new simple nucleoside synthesis Tetrahedron. Lett. 36 7845
Niedballa U and Vorbruggen H 1970 A General Synthesis of Pyrimidine Nucleosides Angew. Chem Int. Ed. Engl. 9 461
Robins M J and Hatfield P W 1982 Nucleic acid related compounds. 37. Convenient and high-yield syntheses of N-[(2-hydroxyethoxy)methyl] heterocycles as “acyclic nucleoside” analogues Can. J. Chem. 60 547
El Mansouri A, Zahouily M and Lazrek H B 2019 HMDS/KI a simple, a cheap and efficient catalyst for the one-pot synthesis of N-functionalized pyrimidines Synth. Commun. 49 1802 and references cited therein
Babkova D A, Chizhovb A O, Khandazhinskayac A L, Coronad A, Espositod F, Tramontanod E, Seley-Radtke K L and Novikova M S 2015 An Efficient Route to Novel Uracil-Based Drug-Like Molecules Synthesis 47 1413 and references cited therein
Manfredini S, Baraldi P G, Bazzanini R, Guarneri M and Simoni D 1994 A new direct glycosylation of pyrimidine, pyrazole, imidazole and purine heterocycles via their N-tetrahydropyranyl (THP) derivatives J. Chem. Soc., Chem. Commun. 5 583
Robins M J, Hatfield P W 1982 Nucleic acid related compounds 37 Convenient and high-yield syntheses of N-[2 hydroxyethoxy) methyl] heterocycles as acylic nucleoside analogues Can. J. Chem. 60 547
El Ashry E S H, El Kilany Y 1997 Acyclonucleosides: Part 1 Seco- Nucleosides Adv. Heterocycl. Chem. 68 1
El Ashry E S H, El Kilany Y 1997 Acyclonucleosides Part III tri-, tetra-, and pentaseco-Nucleosides Adv. Heterocycl. Chem. 69 129
Duehohm K L, Egholm M, Behrens C, Christensen L, Hansen H F, Vulpius T and Peterson K H 1994 Synthesis of peptide nucleic acid monomers containing the four natural nucleobases: thymine, cytosine, adenine, and guanine and their oligomerization J. Org. Chem. 59 5767
Zhou P, Dragulescu-Andrasi A, Bhattacharya B, O’Keefe H, Vatta P, Hyldig-Nielsenb J J and Ly D H 2006 Synthesis of cell-permeable peptide nucleic acids and characterization of their hybridization and uptake properties Bioorg. Med. Chem. Lett. 16 4931
Singh H, Aggarwal P and Kumar S 1990 A Facile Synthesis of 1-Monosubstituted and Unsymmetrically 1,3-Disubstituted Uracils Synthesis 06 520
Lazrek H B, Taourirte M, Oulih T, Barascut J L, Imbach J L, Pannecouque C, Witvrouw M and De Clercq E 2001 Synthesis and anti-HIV activity of new modified 1,2,3-triazole acyclonucleosides Nucleos. Nucleot. Nucleic. Acids 20 1949
El-Zayata W A, El-Sayedb W A and Abdel-Rahmana AA-H 2009 Anti-Hepatitis B Virus Activity of New Pyrimidine and Adenine Peptide Nucleic Acid Analogues Naturforsch. Z. 64c 6
Ali O M, Amer H H and Abdel-Rahman A A H 2007 Synthesis and antiviral evaluation of sugar uracil-1-ylmethylhydrazones and their oxadiazoline derivatives Synthesis 28 2823
Uhlmann E, Peyman A, Breipohl G and Will D W 1998 PNA: Synthetic Polyamide Nucleic Acids with Unusual Binding Properties Angew. Chem. Int. Ed. 37 2796
Alahiane A, Taourirte M, Rochdi A, Redwane N, Sebti S, Engels J W and Lazrek H B 2003 Building blocks for polyamide nucleic acids: Facile synthesis using potassium fluoride doped natural phosphate as basic catalyst Nucleos. Nucleot. Nucleic. Acids 22 109
Krstulović L, Ismaili H, Bajić M, Višnjevac A, Glavaš-Obrovac L and Žinić B 2012 Synthesis of Novel Aliphatic N-sulfonylamidino Thymine Derivatives by Cu(I)-catalyzed Three-component Coupling Reaction Croat. Chem. Acta 85 525
Priego E M, Camarasa M J and Pérez-Pérez M J 2001 Efficient Synthesis of N-3-Substituted 6-Aminouracil Derivatives via N 6-[(Dimethylamino)methylene] Protection Synthesis 3 478
Hovinen J 1997 Selective N3- and 5′-O-Alkylation of 2′,3′-O-isopropylideneuridine with methyl iodide Helv. Chim. Acta 80 851
Bram G, Decodts G, Bensaid Y, Farnoux C C, Galons H and Miocque M 1985 N-Alkylation of Pyrimidine and Purine Derivatives (Uracils, Xanthines, Adenine) using Solid/Liquid Phase-Transfer Catalysis without Solvent Synthesis 5 543
Elayadi H, Mesnaoui M, Korba B E, Smietana M, Vasseur J J, Secrist J A and Lazrek H B 2012 Preparation of 1,4-disubstituted-1,2,3-triazolo ribonucleosides by Na2CuP2O7 catalyzed azide-alkyne 1,3-dipolar cycloaddition ARKIVOC. viii 76
Elayadi H, Smietana M, Vasseur J J, Balzarini J and Lazrek H B 2013 Synthesis of 1,2,3-Triazolyl Nucleoside Analogs as Potential Anti-Influenza A (H3N2 Subtype) Virus Agents Arch. Pharm. Chem. Life. Sci. 347 134
Elayadi H, Ait Ali M, Mehdi A and Lazrek H B 2012 Nanoscrystalline CuO: Synthesis and application as an efficient catalyst for the preparation of 1,2,3-triazole acyclic nucleosides via 1,3-dipolar cycloaddition Catal. Commun. 26 155
Berrocal T, Mesa J L, Larrea E and Arrieta J M 2014 Crystal structure of (NH4)2[FeII 5(HPO3)6], a new open-framework phosphite Acta Crystallogr. Sect. E E70 309
Contreras C A, Sugita S and Ramos E 2006 Preparation of sodium aluminate from basic aluminum sulfate Adv. Tech. Mater. Mater. Proc. J. 8 122
Wang X and Pan Z 2017 Chemical changes and reaction mechanism of hardened cement paste–(NH4)2SO4–H2O system Constr. Build. Mater. 152 434
Zhang B, Ren J, Liu X, Guo Y, Guo Y l, Lu G Z and Wang Y 2010 Novel sulfonated carbonaceous materials from p-toluenesulfonic acid/glucose as a high-performance solid-acid catalyst Cat. Com. 11 629
Novikov M S and Ozerov A A 2005 The Silyl Method for the Synthesis of 1[-2(Phenoxy)ethyl]uracils Chem. Heterocycl. Compd. 41 905
Kelley J L, Baker B R 1982 Irreversible enzyme inhibitors. 202. Candidate active-site-directed irreversible inhibitors of 5-fluoro-2’-deoxyuridine phosphorylase from Walker 256 rat tumor derived from 1-benzyl-5-(3-ethoxybenzyl)uracil J. Med. Chem. 25 600
Zhang Q J, Sun J S, Zhu Y G, Zhang F Y and Yu B 2011 An Efficient Approach to the Synthesis of Nucleosides: Gold(I)-Catalyzed N-Glycosylation of Pyrimidines and Purines with Glycosyl ortho-Alkynyl Benzoates Angew. Chem. Int. Ed. 50 4933
Framski G, Gdaniec Z, Gdaniec M and Boryski J 2006 A reinvestigated mechanism of ribosylation of adenine under silylating conditions Tetrahedron 62 10123
Ganesh A 2013 Potential biological activity of 1,4-sustituted1h-[1,2,3] triazoles Int. J. Chem. Sci. 11 573
Amblard F, Cho J H, Schinazi RF 2009 Cu(I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry Chem. Rev. 109 4207
Kaoukabi H, Kabri Y, Curti C, Taourirte M, Rodriguez-Ubis J C, Snoeck R, Andrei G, Vanelle P and Lazrek H.B 2018 Dihydropyrimidinone/1,2,3-triazole hybrid molecules: Synthesis and anti-varicella-zoster virus (VZV) evaluation Eur. J. Med. Chem. 155 772
Trakossas S, Coutouli-Argyropoulou E and Hadjipavlou-Litina D J 2011 Synthesis of modified triazole nucleosides possessing one or two base moieties via a click chemistry approach Tetrahedron Lett. 52 1673
Elayadi H, Smietana M, Pannecouque C, Leyssen P, Neyts J, Vasseur J-J and Lazrek H B 2010 Straightforward synthesis of triazoloacyclonucleotide phosphonates as potential HCV inhibitors Bioorg. Med. Chem. Lett. 20 7365
Głowacka I E, Balzarini J and Wróblewski A E 2012 Design, synthesis, antiviral, and cytotoxic evaluation of novel phosphonylated 1,2,3-triazoles as acyclic nucleotide analogues Nucleos. Nucleot. Nucleic Acids 31 293
Acknowledgement
The authors would like to acknowledge Prof. Mahmoud H. el Kouni (Department of Pharmacology & Toxicology, the University of Alabama at Birmingham, USA) for helpful discussions. We also thank Professor Marcus Wright (Wake Forest University, North Carolina, USA) for technical assistance and the technical staff of the CAC (Centre of Analysis and Characterization) University Cadi Ayyad for running the spectroscopic analysis.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
BELKHARCHACH, S., IGHACHANE, H., LACHGAR, A. et al. Efficient and selective catalytic N-Alkylation of pyrimidine by ammonium Sulfate@Hydro-thermal carbone under eco-friendly conditions. J Chem Sci 132, 78 (2020). https://doi.org/10.1007/s12039-020-01776-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-020-01776-3