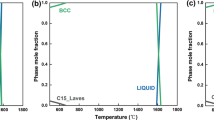
Abstract
This paper deals with hydrogen storage properties of Ti-V based BCC solid solution incorporated with Fe. The alloy with composition Ti2FeV was prepared by arc melting method. X-ray diffraction (XRD) and energy dispersive X-ray analysis studies confirmed formation of solid solution phase with uniform composition and BCC structure. SEM studies revealed the formation of irregular shaped particles with size in the range of few microns up on hydrogenation of the parent alloy. The alloy shows maximum hydrogen storage capacity of 3.41 wt.% at 20 bar and 303 K and the thermodynamic parameters established near room temperature suitability of the alloy for solid state hydrogen storage applications. Hydrogenation kinetics is found to be quite fast and detailed kinetic analysis were done to underscore the hydrogenation mechanism. Activation energy during the initial stage of hydrogenation is found to be 30.8 kJ/mol. The value decreases to 14.4 kJ/mol for extended duration of hydrogenation, and this is explained based on difference in rate determining steps existing at different time scales.
Graphic abstract
Extent of hydrogen absorption as a function of temperature and time for Ti2FeV alloy.








Similar content being viewed by others
References
Jain I P 2009 Hydrogen the fuel for 21st century Int. J. Hydrog. Energy 34 7368
Reardon H, Hanlon J M, Hughes R W, Godula-Jopek A, Mandal T K and Gregory D H 2012 Emerging concepts in solid-state hydrogen storage: the role of nanomaterials design Energy Environ. Sci. 5 5951
Zhao X Y, Ma L Q, Yao Y, Ding Y and Shen X D 2010 Ti2Ni alloy: a potential candidate for hydrogen storage in nickel/metal hydride secondary batteries Energy Environ. Sci. 3 1316
Banerjee S, Kumar A and Pillai C G S 2014 Improvement on the hydrogen storage properties of ZrFe2 Laves phase alloy by vanadium substitution Intermetallics 51 30
Hardian R, Pistidda C, Chaudhary A –L, Capurso G, Gizer G, Cao H, Milanese C, Girella A, Santoru A, Yigit D, Dieringa H, Kainer K U, Klassen T and Dornheim M 2018 Waste Mg-Al based alloys for hydrogen storage Int. J. Hydrog. Energy 43 16738
Young K, Nei J, Huang B and Fetcenko M A 2011 Studies of off-stoichiometric AB2 metal hydride alloy: Part 2. Hydrogen storage and electrochemical properties Int. J. Hydrog. Energy 36 11146
Rogulski Z, Dłubak J, Karwowska M, Krebs M, Pytlik E, Schmalz M, Gumkowska A and Czerwińskia A 2010 Studies on metal hydride electrodes containing no binder additives J. Power Sources 195 7517
Okada M, Kuriiwa T, Tamura T, Takamura H and Kamegawa A 2002 Ti–V–Cr b.c.c. alloys with high protium content J. Alloys Compd. 330–332 511
Seo C-Y, Kim J-H, Lee P S and Lee J-Y 2003 Hydrogen storage properties of vanadium-based b.c.c. solid solution metal hydride J. Alloys Compd. 348 252
Liu X P, Cuevas F, Jiang L J, Latroche M, Li Z N and Wang S M 2009 Improvement of the hydrogen storage properties of Ti-Cr-V-Fe BCC alloy by Ce addition J. Alloys Compd. 476 403
Basak S, Shashikala K, Sengupta P and Kulshreshtha S K 2007 Hydrogen absorption properties of Ti–V–Fe alloys: effect of Cr substitution Int. J. Hydrog. Energy 32 4973
Iba H and Akiba E 1997 Hydrogen absorption and modulated structure in Ti-V-Mn alloys J. Alloys Compd. 253-254 21
Kumar A, Shashikala K, Banerjee S, Nuwad J, Das P and Pillai C G S 2012 Effect of cycling on hydrogen storage properties of Ti2CrV alloy Int. J. Hydrog. Energy 37 3677
Santos S F and Huot J 2009 Hydrogen storage in TiCr1.2(FeV)x BCC solid solutions J. Alloys Compd. 472 247
Ruz P, Kumar A, Banerjee S, Meena S S and Pillai C G S 2014 Hydrogen absorption-characteristics and Mössbauer spectroscopic study of Ti0.67Nb0.33-xFex (x = 0.00, 0.13, 0.20) alloys J. Alloys Compd. 585 120
Ruz P, Banerjee S, Halder R, Kumar A and Sudarsan V 2017 Thermodynamic, Kinetic and microstructural evolution of Ti0.43Zr0.07Cr0.25V0.25 alloy upon hydrogenation Int. J. Hydrog. Energy 42 11482
Qian L, Chou K-C, Lin Q, Jiang L-J and Zhan F 2004 Hydrogen absorption and desorption kinetics of Ag–Mg–Ni alloys Int. J. Hydrogen Energy 29 843
Khawam A and Flanagan D R 2006 Solid-state kinetic models: basics and mathematical fundamentals J. Phys. Chem. B 110 17315
Ruz P and Sudarsan V 2015 An investigation of hydriding performance of Zr2-xTixNi (x = 0.0, 0.3, 0.7, 1.0) alloys J. Alloys Compd. 627 123
Ginstling A M and Brounshtein B I 1950 On diffusion kinetics in chemical reactions taking place in spherical powder grains Zh. Prikl. Khim. 23 1249
Carter R E 1961 Kinetic model for solid-state reactions J. Chem. Phys. 34 2010
Brown P W 1989 Effects of particle size distribution on the kinetics of hydration of tricalcium silicate J. Am. Ceram. Soc. 72 1829
Author information
Authors and Affiliations
Corresponding authors
Additional information
Special Issue on Materials Chemistry
Rights and permissions
About this article
Cite this article
Das, T.K., Kumar, A., Ruz, P. et al. Hydrogen storage properties of Ti2FeV BCC solid solution. J Chem Sci 131, 98 (2019). https://doi.org/10.1007/s12039-019-1674-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-019-1674-x