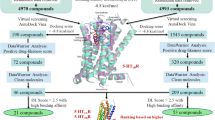
Abstract
A new group of serotonin reuptake inhibitors containing 1,2-dihydrocyclopenta[b]indol-3(4H)-one scaffold was synthesized, starting from indole 5-((1H-indol-3-yl)(1,3-dioxane-4,6-diones as a key intermediates. Following three transformations including intramolecular cyclization and formation of imines, a series of new ligand for human serotonin transporter was obtained. The ability of these ligands to inhibit human TS3 serotonin transporter as well as selectivity toward human D3 dopamine receptor and dopamine transporter were tested in silico using docking software.
Graphical Abstract
SYNOPSIS A series of new serotonin reuptake inhibitors containing 1,2-dihydrocyclopenta[b]indol-3(4H)-one scaffold were designed and synthesized. Affinity to human TS3 transporter and D3 receptor were tested in silico.









Similar content being viewed by others
References
Humber L G, Ferdinandi E, Demerson C A, Ahmed S, Shah U, Mobilio D, Sabatucci J, De Lange B and Labbadia F 1998 Etodolac, a novel antiinflammatory agent. The syntheses and biological evaluation of its metabolites J. Med. Chem. 31 1712
Dejaco Ch, Duftner Ch and Schirmer M 2007 Lack of influence of body mass index on efficacy and tolerance of acemetacin in short-term treatment of musculoskeletal diseases Rheumatol. Int. 27 351
Hughes P, DeVirgilio J, Humber L G, Weichman B and Neuman G 1989 Synthesis and biological evaluation of 4,6-diethyl-1,3,4,5-tetrahydropyrano[4,3-b]indole-4-acetic acid, an isomer of etodolac J. Med. Chem. 32 2134
Lione A and Scialli A R 1995 The developmental toxicity of indomethacin and sulindac Reprod. Toxicol. 9 7
Bellamy N 1997 Etodolac in the management of pain: a clinical review of a multipurpose analgesic Inflammopharmacology 5 139
Affonso V Bizzo H Lage C and Sato A 2009 Influence of growth regulators in biomass production and volatile profile of in vitro plantlets of Thymus vulgaris L J. Agric. Food Chem. 57 6392
Abel S 2007 Auxin Is Surfacing ACS Chem. Biol. 2 380
Michnovicz J J 1991 Altered estrogen metabolism and excretion in humans following consumption of indole-3-carbinol Nutr. Cancer 16 59
Michnovicz J J 1997 Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans Natl. Cancer Inst. 89 718
Holt D A, Yamashita D S, Konialian-Beck A L, Luengo J I, Abel A D, Bergsma D J, Brandt S M and Levy M A 1995 Benzophenone- and indolecarboxylic acids: potent Type-2 specific inhibitors of human steroid 5a-reductase J. Med. Chem. 38 13
Boggs S D, Catalano J G, Gudmundsson K S, Richardson L D and Sebahar P R 2006 Novel cycloalkyl condensed Indoles U. S. Patent 0281804 A1
Baganz N L and Blakely R D A 2013 Dialogue between the immune system and brain, spoken in the language of serotonin ACS Chem. Neurosci. 4 48
Angoa-Pérez M, Kane M J, Briggs D I, Herrera-Mundo N, Sykes C E, Francescutti D M and Kuhn D M 2014 Mice genetically depleted of brain serotonin do not display a depression-like behavioral phenotype ACS Chem. Neurosci. 5 908
Kochanowska-Karamyan A J and Hamann M T 2010 Marine Indole alkaloids: potential new drug leads for the control of depression and anxiety Chem. Rev. 110 4489
Ikarashi Y, Sekiguchi K and Mizoguchi K 2017 Serotonin receptor binding characteristics of Geissoschizine methyl ether, an indole alkaloid in Uncaria Hook Cur. Med. Chem. 24 1
Vangveravong S, Kanthasamy A, Lucaites V L, Nelson D L and Nichols D E 1998 Synthesis and serotonin receptor affinities of a series of trans-2-(Indol-3-yl)cyclopropylamine derivatives J. Med. Chem. 41 4995
Wong D T, Horng J S, Bymaster F P, Hauser K L and Molloy B B 1974 A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-Trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine Life Sci. 15 471
Wong D T, Bymaster F P and Engleman E A 1995 Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication Life Sci. 57 411
Sanchez C, Reines E H and Montgomery S A 2014 A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike? Int. Clin. Psychopharmacol. 29 185
Al-Harbi K S 2012 Treatment-resistant depression: therapeutic trends, challenges, and future directions Patient Prefer. Adherence. 6 369
Nischal A, Tripathi A, Nischal A and Trivedi J K 2012 Suicide and antidepressants: what current evidence indicates Mens Sana Monogr. 10 33
Pompili M, Serafini G, Innamorati M, Ambrosi E, Giordano G, Girardi P, Tatarelli R and Lester D 2010 Antidepressants and suicide risk: a comprehensive overview Pharmaceuticals 3 2861
Porter R A and Vimal M 1995 Teterahydricarbazole derivatives as 5-HT1-like agonists EP 0674622 B1
Bromidge S M 2000 Indole Derivatives as 5-HT Receptor Antagonist U. S. Patent 6028085
Oikawa Y, Hirasawa H and Yonemitsu O 1978 Meldrum’s acid in organic synthesis. A convenient one-pot synthesis of ethyl indolepropionates. A convenient one-pot synthesis of ethyl indolepropionates Tetrahedron Lett. 20 1759
Armstrong E L, Grover H K and Kerr M A 2013 Scandium triflate-catalyzed nucleophilic additions to indolylmethyl meldrum’s acid derivatives via a gramine-type fragmentation: synthesis of substituted indolemethanes J. Org. Chem. 78 10534
Lü Ch, Wang J, Liu Y, Shan W, Sun Q and Shi L 2017 A combination of green solvent and ultrasonic irradiation promotes the catalyst-free reaction of aldehydes, indoles and Meldrum’s acid Res. Chem. Intermed. 43 943
Adamo M F A and Konda V R 2007 Multicomponent synthesis of 3-indolepropionic acids Org. Lett. 9 303
Vickerman K L and Stanley L M 2017 Catalytic, enantioselective synthesis of polycyclic nitrogen, oxygen, and sulfur heterocycles via Rh-catalyzed alkene hydroacylation Org. Lett. 19 5054
Trott O and Olson A J 2010 AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31 455
Coleman J A, Green E M and Gouaux E 2016 X-ray structure of the ts3 human serotonin transporter complexed with paroxetine at the central site Nature 532 334
Chien E Y, Liu W, Zhao Q, Katritch V, Han G W, Hanson M A, Shi L, Newman A H, Javitch J A, Cherezov V and Stevens R C 2010 Structure of the human dopamine d3 receptor in complex with a d2/d3 selective antagonist Science 330 1091
Wang K H, Penmatsa A and Gouaux E 2015 Neurotransmitter and psychostimulant recognition by the dopamine transporter Nature 521 322
Gore S, Baskaran S and König B 2012 Fischer indole synthesis in low melting mixtures Org. Lett. 14 4568
Würtz S, Rakshit S, Neumann J J, Dröge T and Glorius F 2008 Palladium-catalyzed oxidative cyclization of N-aryl enamines: from anilines to indoles Angew. Chem. Int. Ed. 47 7230
Iida H, Yuasa Y and Kibayashi 1980 Intramolecular cyclization of enaminones involving arylpalladium complexes synthesis of carbazoles J. Org. Chem. 45 2938
Janreddy D, Kavala V, Bosco J W, Kuo Ch- and Yao Ch-F 2011 An easy access to carbazolones and 2,3-disubstituted indoles Eur. J. Org. Chem. 12 2360
Bunce R A and Nammalwar B 2009 1,2,3,9-Tetrahydro-4H-carbazol-4-one and 8,9-dihydropyrido-[1,2-a]indol-6(7H)-one from 1H-indole-2-butanoic acid J. Heterocycl. Chem. 46 172
Mishra S, Liu J and Aponick A 2017 Enantioselective Alkyne Conjugate Addition Enabled by Readily Tuned Atropisomeric P,N-Ligands J. Am. Chem. Soc. 139 3352
Najda E, Zakaszewska A, Janikowska K and Makowiec S 2016 Practical method for the preparation of 2,2-dimethyl-5-[aryl(heteroaryl)methyl]-1,3-dioxane-4,6-diones - Synthesis and mechanistic study Synthesis 48 3589
Najda-Mocarska E, Zakaszewska A, Janikowska K and Makowiec S 2018 New thiourea organocatalysts and their application for the synthesis of 5-(1H-indol-3-yl)methyl-2,2-dimethyl-1, 3-dioxane-4,6-diones a source of chiral 3-indoylmethyl ketenes Synth. Commun. 48 14
Fillion E, Fishlock D, Wilsily A and Goll J M 2005 Meldrum’s acids as acylating agents in the catalytic intramolecular Friedel-Crafts reaction J. Org. Chem. 70 1316
Fillion E and Fishlock D 2003 Convenient access to polysubstituted 1-indanones by Sc(OTf)\(_3\)-catalyzed intramolecular Friedel-Crafts acylation of benzyl Meldrum’s acid derivatives Org. Lett. 5 4653
Prajakta N N, Nabil A H A and Radhika S K 2015 Beckmann rearrangement for the synthesis of derivatives of \(\upbeta \)- and \(\upgamma \)-carbolinones, dihydropyrrolopyridinone and tetrahydroisoquinolinone ARKIVOC 7 362
Banwell J M G and Smith J A 2002 Exploiting multiple nucleophilic sites on pyrrole for the assembly of polyheterocyclic frameworks: application to a formal total synthesis of (\(\pm )\)-aspidospermidine Chem. Soc. Perkin Trans. 10 2613
Pamukcu R and Piazza G A 2002 Method of inhibiting neoplastic cells with indole derivatives U.S. Patent 6358992 B1
Maertens F, Toppet S, Hoornaert G J and Compernolle F 2005 Incorporation of an indole-containing diarylbutylamine pharmacophore into furo[2,3-a]carbazole ring systems Tetrahedron 61 1715
Haddad M, Dorbais J and Larcheveque M 1997 Stereocontrolled reductive amination of 3-hydroxy ketones Tetrahedron Lett. 38 5981
Cherukupally P, Vajrala V R, Adla V K, Rangineni S and Kanniah S 2011 Preparation of rasagiline salts therof U.S. Patent 20110218360 A1
Kang S, Cooper G, Dunne S F, Luan C, Surmeier D J and Silverman R B 2013 Structure–activity relationship of N,N’-disubstituted pyrimidinetriones as Ca 1.3 calcium channel-selective antagonists for Parkinson’s disease J. Med. Chem. 56 4786
Barluenga J, Jimenez-Aquino A, Aznar F and Valdes C 2009 Modular Synthesis of indoles from imines and o-Dihaloarenes or o-Chlorosulfonates by a Pd-catalyzed cascade process J. Am. Chem. Soc. 131 4031
Acknowledgements
The project was carried-out within the PARENT-BRIDGE programme of the Foundation for Polish Science (POMOST/2013-8/6), co-financed from the European Union under the European Regional Development Fund. We warmly thank undergraduate and graduate students Mateusz Leśniewski and Marek Cichon for their contribution to the project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Szewczyk, M., Punda, P., Janikowska, K. et al. Design, synthesis, and molecular docking of new 5-HT reuptake inhibitors based on modified 1,2-dihydrocyclopenta[b]indol-3(4H)-one scaffold. J Chem Sci 131, 45 (2019). https://doi.org/10.1007/s12039-019-1621-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-019-1621-x