Abstract
The crystal structure of the compound, Zn(II) 5,10,15,20-tetrakis(meta-methoxyphenyl)porphyrin chloroform trisolvate, \([\hbox {ZnT}(m\hbox {-OCH}_{3})\hbox {PP}]{\cdot } 3\hbox {CHCl}_{3}\) 1 reveals that it forms a weak one-dimensional chain structure through interaction between Zn of porphyrin and the oxygen atom of the methoxy group of a neighbouring porphyrin. The zinc–oxygen interaction observed in compound 1 is compared with Zn(II) 5,10,15,20-tetrakis(para-methoxyphenyl)porphyrin \([\hbox {ZnT}(p\hbox {-OCH}_{3})\hbox {PP}]\) 2 and Zn(II) 5,10,15,20-tetrakis(3,4,5-tri-methoxyphenyl)porphyrin \([\hbox {ZnT}(3,4,5\hbox {-triOCH}_{3})\hbox {PP}]\) 3 to understand the preferred methoxy-position of interaction. The strength of the non-covalent zinc–oxygen (methoxy group of a neighboring porphyrin) interaction in compound 1 is in between that of similar interactions observed in compounds 2 and 3. The Mulliken charge analysis using theoretical calculation at the DFT level shows that the meta-methoxy oxygen has a higher probability of binding to the metal than the para-methoxy oxygen. In the presence of nucleophiles, the formation of one-dimensional chain structure stops due to the binding of the nucleophiles to the metal zinc. The photoluminescence and differential scanning calorimetric studies were also performed for compound 1.
Graphical Abstract
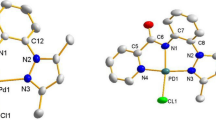
SYNOPSIS A zinc porphyrin \([\hbox {ZnT}(m\hbox {-OCH}_{3})\hbox {PP}]{\cdot } 3\hbox {CHCl}_{3}\) 1 has been synthesized and structurally characterized using single crystal X-ray diffraction and other spectroscopic methods. The crystal structure of 1 shows a weak interaction between Zn of porphyrin and the oxygen atom of the methoxy group of a neighbouring porphyrin. The Zn-oxygen interaction observed in compound 1 is compared with Zn-oxygen interaction present in other methoxy-substituted porphyrins \([\hbox {ZnT}(p\hbox {-OCH}_{3})\hbox {PP}]\) 2 and [ZnT(3,4, 5-\(\hbox {triOCH}_{3})\hbox {PP}\)] 3.







Similar content being viewed by others
References
Huaping L, Bing Z, Yi L, Lingrong G, Wei W, Fernando K A S, Kumar S, Lawrence F A and Sun Y P 2004 Selective interactions of porphyrins with semiconducting single-walled carbon nanotubes J. Am. Chem. Soc. 126 1014
Urbanova M, Setnic V, Kral V and Volka K 2001 Noncovalent interactions of peptides with porphyrins in aqueous solution: conformational study using vibrational CD spectroscopy J. Pept. Sci. 60 307
Murakami H, Nomura T and Nakashima N 2003 Noncovalent porphyrin-functionalized single-walled carbon nanotubes in solution and the formation of porphyrin–nanotube nanocomposites Chem. Phys. Lett. 378 481
Cheng F, Zhang S, Adronov A, Echegoyen L and Diederich F 2006 Triply fused \(\text{ Zn }^{II}\)–porphyrin oligomers: synthesis, properties, and supramolecular interactions with single-walled carbon nanotubes (swnts) Chem. Eur. J. 12 6062
Titi H M, Tripuramallu B K and Goldberg I 2016 Porphyrin-based assemblies directed by non-covalent interactions highlights of recent investigations CrystEngComm 18 3318
Stangel C, Charisiadis A, Zervaki G E, Nikolaou V, Charalambidis G, Kahnt A, Rotas G, Tagmatarchis N and Coutsolelos G A 2017 A case study for artificial photosynthesis: non-covalent interactions between \({\rm C}_{60}\) dipyridyl and zinc porphyrin dimer J. Phys. Chem. 121 4850
Borah K D and Bhuyan J 2017 Magnesium porphyrins with relevance to chlorophylls Dalton Trans. 46 6497
Hayashi T and Ogoshi H 1997 Molecular modelling of electron transfer systems by noncovalently linked porphyrin–acceptor pairing Chem. Soc. Rev. 26 355
Lang K, Mosinger J and Wagnerov D M 2004 Photophysical properties of porphyrinoid sensitizers non-covalently bound to host molecules; models for photodynamic therapy Coord. Chem. Rev. 2 48 321
Bhuyan J and Sarkar S 2012 Nitrous acid mediated synthesis of iron-nitrosyl-porphyrin: pH-dependent release of nitric oxide Chem. Asian J. 7 2690
Batten S R, Champness N R, Chen X, Martinez J G, Kitagawa S, Ohrstrom L, O’Keeffe M, Suhh M P and Reedijk J 2012 Coordination polymers, metal–organic frameworks and the need for terminology guidelines CrystEngComm 14 3001
Huh S, Kim S J and Kim Y 2016 Porphyrinic metal-organic frameworks from custom-designed Porphyrins CrystEngComm 18 345
Gao W Y, Chrzanowski M and Ma S 2014 Metal–metalloporphyrin frameworks: a resurging class of functional materials Chem. Soc. Rev. 43 5841
Lipstman S and Goldberg I 2010 Porphyrin framework solids. Hybrid supramolecular assembly modes of tetrapyridylporphyrin and aqua nitrates of lanthanoid ions Cryst.Growth Des. 10 1823
Johnson J A, Lin Q, Wu L C, Obaidi N, Olson Z L, Reeson R T, Chen Y S and Zhang J 2013 A “pillar-free”, highly porous metalloporphyrinic framework exhibiting eclipsed porphyrin arrays Chem. Comm. 49 2828
Sun W, Wang H, Qi D, Wang L, Wang K, Kan J, Li W, Chen Y and Jiang J 2012 5,10,15,20-tetra(4-pyridyl)porphyrinato zinc coordination polymeric particles with different shapes and luminescent properties CrystEngComm 14 7780
Krupitsky H, Stein Z and Goldberg I 1994 Crystalline complexes, coordination polymers and aggregation modes of tetra(4-pyridyl)porphyrin J. Incl. Phenom. Macrocycl. Chem. 18 177
Shmilovits M, Vinodu M and Goldberg I 2004 Coordination polymers of tetra(4 carboxyphenyl)porphyrins sustained by tetrahedral zinc ion linkers Cryst. Growth Des. 4 633
Teo T L, Vetrichelvan M and Lai Y H 2003 Infinite three-dimensional polymeric metalloporphyrin network via six-coordinate Zn(II) and two axial oxygen donors Org. Lett. 5 4207
Ikbal S, Brahma S, Dhamija A and Rath S P 2014 Building-up novel coordination polymer with Zn(II) porphyrin dimer: Synthesis, structures, surface morphology and effect of axial ligands J. Chem. Sci. 126 1451
Bhuyan J and Sarkar S 2011 Self-assembly of magnesium and zinc trimethoxyphenylporphyrin polymer as nanospheres and nanorods Cryst. Growth Des. 11 5410
Saleh R Y and Straub D K 1989 13C NMR spectra of tetra(3,4,5-trimethoxyphenyl)porphyrin and its zinc and iron(III) complexes Inorg. Chim. Acta 156 9
McGill S, Nesterov V N and Gould S L 2013 [5,10,15,20-Tetra-kis(4-methoxyphenyl)porphyrinato]zinc dichloro-methane disolvate Acta Cryst. 69 m471
Okada S and Segawa H 2003 Substituent-control exciton in j-aggregates of protonated water-insoluble porphyrins J. Am. Chem. Soc. 125 2792
Wei X, Du X, Chen D and Chen Z 2006 Thermal analysis study of 5,10,15,20-tetrakis (methoxyphenyl)porphyrins and their nickel complexes Thermochim. Acta 440181
Dolomanov O V, Bourhis L J, Gildea R J, Howard J A K and Puschmann H 2009 Complete structure solution, refinement and analysis program J. Appl. Cryst. 42 339
Adler A D, Longo F R, Finarelli J D, Goldmacher J, Assour J and Korsakoff L 1967 A simplified synthesis for meso-tetraphenylporphine J. Org. Chem. 32 476
Goldberg I, Krupitsky H, Stein Z, Hsiou Y and Strouse E C 1994 Supramolecular architectures of functionalized tetraphenylmetalloporphyrins in crystalline solids. Studies of the 4-methoxyphenyl, 4-hydroxyphenyl and 4-chlorophenyl derivatives Supramol. Chem. 4203
Barnnet G H, Hudson M F and Smith K M 1975 Concerning meso-tetraphenylporphyrin purification J. Chem. Soc. 14 1401
Gouterman M 1961 Spectra of Porphyrins J. Mol. Spectrosc. 6 138
Zaitzeva S V, Zdanovich S A, Ageeva T A, Ocheretovi A S and Golubchikov O A 2000 Influence of the nature of porphyrin and extraligand on the stability of zinc extracomplexes Molecules 5 786
Favereau L, Cnossen A, Kelber J B, Gong J Q, Oetterli R M, Cremers J, Herz L M and Anderson H L 2015 Six-coordinate zinc porphyrins for template-directed synthesis of spiro-fused nanorings J. Am. Chem. Soc. 137 14256
Wang S, Peng U, Zhang C, Li Y and Liu C 2016 Synthesis of zinc porphyrn and effect of peripheral substituent on the coordination reaction Indian J. Chem. 55 145
Bhuyan J 2016 Nucleophilic ring-opening of iron(III)-hydroxyisoporphyrin Dalton Trans. 45 2694
Tumer M, Gungor S A and Çiftaslan A R 2016 Solid state and solution photoluminescence properties of a novel meso-meso-linked porphyrin dimer schiff base ligand and its metal complexes J. Lumin. 170 108
Boucher L J and Katz J J 1967 The infared spectra of metalloporphyrins (4000–160 \(\text{ cm }^{-1})\) J. Am. Chem. Soc. 89 1340
Filip A G, Clichici S, Daicoviciu D, Ion R M, Tatomir C, Rogojan L, Opris I, Mocan T, Olteanu D and Muresan A 2011 Possible in vivo mechanisms involved in photodynamic therapy using tetrapyrrolic macrocycles Braz. J. Med. Biol. Res. 44 53
Takashima H, Fujimoto E, Hirai C and Tsukahara K 2008 Synthesis and spectroscopic properties of reconstituted zinc-myoglobin appending a DNA-binding platinum(II) complex Chem. Biodivers. 5 2101
Borah K D, Singh N G and Bhuyan J 2017 Magnesium trimethoxyphenylporphyrin chain controls energy dissipation in the presence of cholesterol J. Chem. Sci. 129 449
Guan C, Li I, Chen D, Gao Z and Sun W 2002 Thermal behavior and thermal decomposition study of porphyrin polymers containing different spacer groups Thermochim. Acta 413 31
Medforth C J, Haddad R E, Muzzi C M, Dooley N R, Jaquinod L, Shyr D C, Nurco D J, Olmstead M M, Smith K M, Ma J G and Shelnutt J A 2003 Unusual aryl-porphyrin rotational barriers in peripherally crowded porphyrins Inorg. Chem. 42 2227
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery Jr J A., Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B,Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Bakken V, Adamo C, Jaramillo J,Gomperts R, Stratmann R E, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, RabuckA D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C and Pople J A Gaussian 03 (Revision B.05), Gaussian 03, Revision B.04, Gaussian Inc., Pittsburgh, P A, 2003
Acknowledgements
JB acknowledges SERB, DST, New Delhi for funding (YSS/2015/000394). Authors thank Dr. Md. Harunar Rashid, Department of Chemistry, Rajiv Gandhi University, Itanagar for allowing to use the fluorescence spectrophotometer.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borah, B.P., Bhuyan, J. Influence of position of methoxy groups in Zn-methoxyphenylporphyrins. J Chem Sci 130, 117 (2018). https://doi.org/10.1007/s12039-018-1516-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1516-2