Abstract
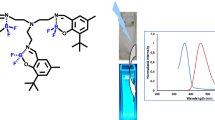
Boranils containing ferrocene and triphenylamine groups were synthesized and well characterized by IR, \(^{1}\hbox {H}\), \(^{13}\hbox {C}\), \(^{11}\hbox {B}\) and \(^{19}\hbox {F}\) NMR and ESI-MS spectrometry. UV-Vis absorption, steady-state and time-resolved fluorescence techniques were employed to study their photophysical properties. The presence of electron rich substituents caused significant alterations in their absorption and emission spectra. As compared to the parent boranil compound (\(\lambda _{\mathrm{abs}} = 342 \hbox { nm}\)), boranil-triphenylamine and boranil-ferrocene conjugates exhibited red shifted absorption maxima (\(\lambda _{\mathrm{abs}} = 384\), 375 nm, respectively). The boranil-triphenylamine and boranil-ferrocene conjugates displayed red shifted emission spectra (\(\lambda _{\mathrm{em}} = 524\), 489 nm, respectively), as compared to the parent boranil (\(\lambda _{\mathrm{em}} = 471 \hbox { nm}\)). Also, large Stokes shifts (\(6217{-}6958 \hbox { cm}^{-1}\)) were observed for both the conjugates in solution phase.
Graphical Abstract
SYNOPSIS Synthesis and optical properties of boranils containing ferrocene and triphenylamine groups are reported. The presence of substituents caused significant bathochromic shifts in their absorption and emission spectra. As compared to the parent boranil compound, boranil-triphenylamine and boranil-ferrocene conjugates exhibited 32–41 nm red shifted absorption spectra, and 18–53 nm red shifted emission spectra in solution. Also, large Stokes shifts (6200–6900 \(\hbox { cm}^{-1}\)) were observed for both the conjugates in solution.

Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.




Similar content being viewed by others
References
Coskun A, Yilmaz M D and Akkaya E U 2007 Bis(2-pyridyl)-Substituted Boratriazaindacene as an NIR-Emitting Chemosensor for Hg(II) Org. Lett. 9 607
Yee M-C, Fas S C, Stohlmeyer M M, Wandless T J and Cimprich K A 2005 A Cell-permeable, Activity-based Probe for Protein and Lipid Kinases J. Biol. Chem. 280 29053
Kobayashi H, Ogawa M, Alford R, Choyke P L and Urano Y 2010 New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging Chem. Rev. 110 2620
Kamkaew A, Lim S H, Lee H B, Kiew L V, Chung L Y and Burgess K 2013 BODIPY dyes in photodynamic therapy Chem. Soc. Rev. 42 77
O’Regan B and Graetzel M 1991 A low-cost, high-efficiency solar cell based on dye-sensitized colloidal \(\text{ TiO }_{2}\) films Nature 353 737
Ryan A, Tuffy B, Horn S, Blau W J and Senge M O 2011 Carbazole-linked porphyrin dimers for organic light emitting diodes: synthesis and initial photophysical studies Tetrahedron 67 8248
Lakshmi V, Sharma R and Ravikanth M 2016 Functionalized boron-dipyrromethenes and their applications Rep. Org. Chem. 6 1
Vedamalai M, Kedaria D, Vasita R, Mori S and Gupta I 2016 Design and synthesis of BODIPY-clickate based \(\text{ Hg }^{2+}\) sensors: the efect of triazole binding mode with \(\text{ Hg }^{2+}\) on signal transduction Dalton Trans. 45 2700
Ziessel R and Harriman A 2011 Artificial light-harvesting antennae: electronic energy transfer by way of molecular funnels Chem. Commun. 47 611
Kesavan P E and Gupta I 2014 Carbazole substituted boron dipyrromethenes Dalton Trans. 43 12405
Kesavan P E, Das S, Lone M Y, Jha P C, Mori S and Gupta I 2015 Bridged bis-BODIPYs: their synthesis, structures and properties Dalton Trans. 44 17209
Kesavan P E, Behera R N, Mori S and Gupta I 2017 Carbazole Substituted BODIPYs: Synthesis, Computational, Electrochemical and DSSC Studies J. Fluoresc. 27 2131
Vedamalai M, Krishnakumar V G, Gupta S, Mori S and Gupta I 2017 Synthesis and characterization of styryl-BODIPY derivatives for monitoring in vitro Tau aggregation Sens. Actuat. B-Chem. 244 673
Balsukuri N, Boruah N J, Kesavana P E and Gupta I 2018 Near Infra-Red Dyes Based on Pyrene Aza-BODIPYs New J. Chem. 42 5875
Balsukuri N, Lone M Y, Jha P C, Mori S and Gupta I 2016 Synthesis, Structure, and Optical Studies of Donor–Acceptor-Type Near-Infrared (NIR) Aza–Boron-Dipyrromethene (BODIPY) Dyes Chem. Asian J. 11 1572
Balsukuri N, Mori S and Gupta I 2016 Donor acceptor type ferrocene substituted aza-BODIPYs: Synthesis, optical and electrochemical studies J. Porphyrins Phthalocyanines 20 720
Ge Y and O’Shea D F 2016 Azadipyrromethenes: from traditional dye chemistry to leading edge applications Chem. Soc. Rev. 45 384618
Gorman A, Killoran J, O’Shea C, Kenna T, Gallagher W M and O’Shea D F 2004 In Vitro Demonstration of the Heavy-Atom Effect for Photodynamic Therapy J. Am. Chem. Soc. 126 10619
Kim H, Burghart A, Welch M B, Reibenspies J and Burgess K 1999 Synthesis and spectroscopic properties of a new 4-bora-3a,4a-diaza-\(s\)-indacene (BODIPY) dye Chem. Commun. 1889
Quin-De L, Mudadu M S, Thummel R, Tao Y and Wang S 2005 From Blue to Red: Syntheses, Structures, Electonic, and Electroluminescent Properties of tunable Luminescent N,N Chelate Boron Complexes Adv. Funct. Mater. 15 143
Jadhav T, Maragani R, Misra R, Sreeramulu V, Rao D N and Mobin S M 2013 Design and synthesis of donor–acceptor pyrazabole derivatives for multiphoton absorption Dalton Trans. 42 4340
Misra R, Jadhav T and Mobin S M 2014 Ferrocenyl pyrazaboles: design, synthesis, structure, and properties Dalton Trans. 43 2013
Massue J, Frath D, Retailleau P, Ulrich G and Ziessel R 2013 Synthesis of Luminescent Ethynyl-Extended Regioisomers of Borate Complexes Based on 2-(2\(^{\prime }\)-Hydroxyphenyl)benzoxazole Chem. Eur. J. 19 5375
Frath D, Azizi S, Ulrich G and Ziessel R 2012 Chemistry on Boranils: An Entry to Functionalized Fluorescent Dyes Org. Lett. 14 4774
Frath D, Azizi S, Ulrich G, Retailleau P and Ziessel R 2011 Facile Synthesis of Highly Fluorescent Boranil Complexes Org. Lett. 13 3414
Berezin M Y and Achilefu S 2010 Fluorescence Lifetime Measurements and Biological Imaging Chem. Rev. 110 2641
Gao H, Li Y, Wang L, Ji C, Wang Y, Tian W, Yanga X and Yin L 2014 High performance asymmetrical push–pull small molecules end-capped with cyanophenyl for solution-processed solar cells Chem. Commun. 50 10251
Rosenblum M, Brawn N, Papenmeier J and Applebaum M 1966 Synthesis of ferrocenylacetylenes J. Organomet. Chem. 7 193
Dhokale B, Jadhav T, Patil Y and Misra R 2016 Symmetrical and unsymmetrical ferrocenyl perylenediimides: Design, synthesis and properties Dyes Pigments 134 164
Dhokale B, Gautam P and Misra R 2012 Donor–acceptor perylenediimide–ferrocene conjugates: synthesis, photophysical, and electrochemical properties Tetrahedron Lett. 53 2352
Kollmannsberger M, Rurack K, Resch-Genger U and Daub J 1998 Ultrafast Charge Transfer in Amino-Substituted Boron Dipyrromethene Dyes and Its Inhibition by Cation Complexation: A New Design Concept for Highly Sensitive Fluorescent Probes J. Phys. Chem. A 102 10211
Mohammed O F and Sarhan A A O 2010 Ultrafast excited-state dynamics of ferrocene-bridge-acceptor system Chem. Phys. 372 17
Acknowledgements
Financial support from SERB (EMR/2015/000779), Govt. of India, is greatly acknowledged. NM thanks IIT Gandhinagar for the fellowship and infrastructural support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue on Modern Trends in Inorganic Chemistry
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manav, N., Tyagi, A., Pandey, V. et al. Ferrocene and triphenylamine appended boranils. J Chem Sci 130, 79 (2018). https://doi.org/10.1007/s12039-018-1490-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1490-8