Abstract
A series of five new donor–acceptor molecules based on imidazo-anthraquinone-amines have been synthesized and characterized. The structure-property relationship in these molecules is systematically examined by absorption-emission spectroscopy, cyclic voltammetry and theoretical studies. Optical properties of these molecules have been studied in solvents of varying polarity as well as in neat solid film and found to be affected by the nature of triarylamine substituent with broad absorption windows, strong charge transfer transitions (425–502 nm), high molar extinction coefficients and emission in green light (500–568 nm). Electrochemical data indicated that the dyes possess relatively low-lying LUMO values (\(-3.18\) to \(-3.42\) eV) while TGA studies revealed good thermal stability. The donor-acceptor architecture and HOMO–LUMO energies were further rationalized using DFT calculations. Experimental studies along with theoretical calculations suggest that these compounds have potential to be used as n-type materials in optoelectronic devices.
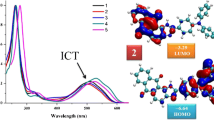
GRAPHICAL ABSTRACT
Imidazoanthraquinone–amine derivatives with donor–acceptor molecular architecture were synthesized using Buchwald–Hartwig amination reaction featuring ICT absorption and low lying LUMO energy level (\(-3.18\) to \(-3.42\) eV). These are comparable with well-known n-type materials for their applications in organic electronics.








Similar content being viewed by others
References
(a) Shirota Y 2000 Organic materials for electronic and optoelectronic devices J. Mater. Chem. 10 1; (b) Shirota Y 2005 Photo and electroactive amorphous molecular materials—molecular design, syntheses, reactions, properties, and applications J. Mater. Chem. 15 75; (c) Koene B E, Loy D E and Thompson M E 1998 Asymmetric Triaryldiamines as Thermally Stable Hole Transporting Layers for Organic Light-Emitting Devices Chem. Mater. 10 2235; (d) Wu C, Djurovich P I and Thompson M E 2009 Study of Energy Transfer and Triplet Exciton Diffusion in Hole-Transporting Host Materials Adv. Func. Mater. 19 3157
(a) Song Y, Di C, Yang X, Li S, Xu W, Liu Y, Yang L, Shuai Z, Zhang D and Zhu D 2006 A Cyclic Triphenylamine Dimer for Organic Field-Effect Transistors with High Performance J. Am. Chem. Soc. 128 15940; (b) Cravino A, Roquet S, Aleveque O, Leriche P, Frere P and Roncali J 2006 Triphenylamine—Oligothiophene Conjugated Systems as Organic Semiconductors for Opto-Electronics Chem. Mater. 18 2584; (c) Saragi T P I, Lieker T F and Salbeck J 2006 Comparison of Charge-Carrier Transport in Thin Films of Spiro-Linked Compounds and Their Corresponding Parent Compounds Adv. Funct. Mater. 16 966
(a) Mishra A, Fischer M K R and Baüerle P 2009 Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules Angew. Chem. Int. Ed. 48 2474; (b) Roncali J, Leriche P and Cravino A 2007 From One-to Three-Dimensional Organic Semiconductors: In Search of the Organic Silicon Adv. Mater. 19 2045
(a) Bordeau G, Lartia R, Metge G, Fiorini-Debuisschert C, Charra F and Teulade-Fichou M.–P 2008 Trinaphthylamines as Robust Organic Materials for Two-Photon-Induced Fluorescence J. Am. Chem. Soc. 130 16836; (b) Bhaskar A, Ramakrishna G, Lu Z, Twieg R, Hales J M, Hagan D J, Van Stryland E and Goodson T 2006 Investigation of Two-Photon Absorption Properties in Branched Alkene and Alkyne Chromophores J. Am. Chem. Soc. 128 11840
(a) Chi L, Wu Y, Zhang X, Ji S, Shao J, Guo H, Wang X and Zhao J 2010 Ethynylated Triphenylamine Monoboronic acid Chemosensors: Experimental and Theoretical Studies J. Fluoresc. 20 1255; (b) Sreenath K T G T and Gopidas K R 2011 Cu(II) Mediated Generation and Spectroscopic Study of the Tris(4-anisyl)amine Radical Cation and Dication. Unusually Shielded Chemical Shifts in the Dication Org. Lett. 13 1134; (c) Ghosh K, MasantaG, Fröhlich R, Petsalakis I D and Theodorakopoulos G 2009 Triphenylamine-Based Receptors in Selective Recognition of Dicarboxylic Acids J. Phys. Chem. B 113 7800
Thelakkat M 2002 Star-Shaped, Dendrimeric and Polymeric Triarylamines as Photoconductors and Hole Transport Materials for Electro-Optical Applications Mater. Eng. 287 442
(a) Shen J-Y, Yang X-L, Huang T-H, Lin J T, Ke T-H, Chen L-Y, Wu C-C and Yeh M-C P 2007 Ambipolar Conductive 2,7-Carbazole Derivatives for Electroluminescent Devices Adv. Funct. Mater. 17 983; (b) Thomas K R J, Velusamy M, Lin J T, Tao Y-T and Chuen C-H 2004 Cyanocarbazole Derivatives for High-Performance Electroluminescent Devices Adv. Funct. Mater. 14 387; (c) Thomas K R J, Lin J T, Tao Y-T and Ko C-W 2001 Light-Emitting Carbazole Derivatives: Potential Electroluminescent Materials J. Am. Chem. Soc. 123 9404; (d) Xia Z Y, Su J H, Fan H H, Cheah K W, Tian H and Cheah C H J 2010 Multifunctional Diarylamine-Substituted Benzo[\(k\)]fluoranthene Derivatives as Green Electroluminescent Emitters and Nonlinear Optical Materials J. Phys. Chem. C 114 11602; (e) Shaikh A M, Sharma B K, Chacko S and Kamble R M 2017 Novel electroluminescent donor–acceptors based on dibenzo[\(a,c\)]phenazine as hole-transporting materials for organic electronics New. J. Chem. 41 628
(a) Meng H, Sun F P, Goldfinger M B, Jaycox G D, Li Z G, Marshall W J and Blackman G S 2005 High-Performance, Stable Organic Thin-Film Field-Effect Transistors Based on Bis-5‘-alkylthiophen-2‘-yl-2,6-anthracene Semiconductors J. Am. Chem. Soc. 127 2406; (b) Chung D S, Park J W, Park, J–H, Moon D, Kim G H, Lee H-S, Lee D H, Shim H-K, Kwon S-K and Park C E 2010 High mobility organic single crystal transistors based on soluble triisopropylsilylethynyl anthracene derivatives J. Mater. Chem. 20 524; (c) Teng C, Yang X, Yang C, Li S, Cheng M, Hagfeldt A and Sun L 2010 Molecular Design of Anthracene-Bridged Metal-Free Organic Dyes for Efficient Dye-Sensitized Solar Cells J. Phys. Chem. C 114 9101; (d) Sharma B K, Shaikh A M and Kamble R M 2015 Synthesis, photophysical, electrochemical and thermal investigation of Triarylamines based on 9\(H\)-Xanthen-9-one: Yellow–green fluorescent materials J. Chem. Sci. 127 2063; (e) Shaikh A M, Sharma B K and Kamble R M 2015 Photophysical, Electrochemical and Thermal Studies of 5-methyl-5\(H\)-Benz[\(g\)]indolo[2,3-\(b\)] quinoxaline Derivatives: Green and Yellow Fluorescent Materials Can. Chem. Trans. 3 158; (f) Shaikh A M, Sharma B K and Kamble R M 2015 Synthesis, Photophysical, Electrochemical and Thermal Studies of Triarylamines based on benzo[\(g\)]quinoxalines J. Chem. Sci. 127 1571; (g) Shaikh A M, Chacko S and Kamble R M 2017 Synthesis, Optoelectronic and Theoretical Investigation of Anthraquinone Amine-Based Donor-Acceptor Derivatives ChemistrySelect. 2 7620
Doi H, Kinoshita M, Okumoto K and Shirota Y 2003 A Novel Class of Emitting Amorphous Molecular Materials with Bipolar Character for Electroluminescence Chem. Mater. 15 1080
Chen C-T, Wei Y, Lin J-S, Moturu M V R K, Chao W-S, Tao Y-T and Chien C-H 2006 Doubly Ortho-Linked Quinoxaline/Diphenylfluorene Hybrids as Bipolar, Fluorescent Chameleons for Optoelectronic Applications J. Am. Chem. Soc. 128 10992
Tao Y, Wang Q, AoL, Zhong C, Yang C, Qin J and Ma D 2010 Highly Efficient Phosphorescent Organic Light-Emitting Diodes Hosted by 1,2,4-Triazole-Cored Triphenylamine Derivatives: Relationship between Structure and Optoelectronic Properties J. Phys. Chem. C 114 601
Ge Z, Hayakawa T, Ando S, Ueda M, Akiike T, Miyamoto H, Kajita T and Kakimoyo M 2008 Novel Bipolar Bathophenanthroline Containing Hosts for Highly Efficient Phosphorescent OLEDs Org. Lett. 10 421
Sharma B K, Shaikh A M, Agarwal N and Kamble R M 2016 Synthesis, photophysical and electrochemical studies of acridone-amine based donor–acceptors for hole transport materials RSC Adv. 6 17129
Shaikh A M, Sharma B K, Chacko S and Kamble RM 2016 Synthesis and opto-electrochemical properties of tribenzo[a,c,i]phenazine derivatives for hole transport materials RSC Adv. 6 94218
Thomas K R J, Lin J T, Tao Y-T and Chuen C-H 2004 New Carbazole—Oxadiazole Dyads for Electroluminescent Devices: Influence of Acceptor Substituents on Luminescent and Thermal Properties Chem. Mater. 16 5437
(a) da Silva Jr E N, Cavalcanti B C, Guimarães T T, Carmo M D, Pinto F R, Cabral I O, Pessoa C, Costa-Lotufo L V, de Moraes M O, de Andrade C K Z, dos Santos M R, de Simone C A, Goulart M O F and Pinto A V 2011 Synthesis and evaluation of quinonoid compounds against tumor cell lines Eur. J. Med. Chem. 46 399; (b) Huang H-S, Chen T-C, Chen R-H, Huang K-F, Huang F-C, Jhan J-R, Chen C-L, Lee C-C, Lo Y and Lin J-J 2009 Synthesis, cytotoxicity and human telomerase inhibition activities of a series of 1,2-heteroannelated anthraquinones and anthra[1,2-\(d\)]imidazole-6,11-dione homologues Bio. Org. Med. Chem. 17 7418
(a) Peng X, Wu Y, Fan J, Tian M and Han K 2005 Colorimetric and Ratiometric Fluorescence Sensing of Fluoride: Tuning Selectivity in Proton Transfer J. Org. Chem. 70 10524; (b) Hernández C M, Figueroa L E S, Moragues M E, Raposo M M M, Batista R M F, Costa S P G, Pardo T, Máñez R M and Sancenón F 2014 Imidazoanthraquinone Derivatives for the Chromofluorogenic Sensing of Basic Anions and Trivalent Metal Cations J. Org. Chem. 79 10752; (c) Saha S, Ghosh A, Mahato P, Mishra S, Mishra S K, Suresh E, Das S and Das A 2010 Specific Recognition and Sensing of \(\text{CN}^{-}\) in Sodium Cyanide Solution Org. Lett. 12 3406; (d) Kumari N, Jha S and Bhattacharya S 2011 Colorimetric Probes Based on Anthraimidazolediones for Selective Sensing of Fluoride and Cyanide Ion via Intramolecular Charge Transfer J. Org. Chem. 76 8215
Sharma B K, Dixit S, Chacko S, Kamble R M and Agarwal N 2017 Synthesis and Studies of Imidazoanthraquinone Derivatives for Applications in Organic Electronics Eur. J. Org. Chem. 30 4389
Li G Y, Zhao G J, Liu Y H, Han K-L and He G–Z 2010 TD-DFT study on the sensing mechanism of a fluorescent chemosensor for fluoride: Excited-state proton transfer J. Comput. Chem. 311 759
Sun S–S and Sariciftci N S 2005 Organic Photovoltaics: Mechanism, Materials and Devices (United States: Taylor and Francis) p.199
(a) Dawson W R and Windsor M W 1968 Fluorescence yields of aromatic compounds. J. Phys. Chem. 72 3251 (b) Hamai S and Hirayama F1983 Actinometric determination of absolute fluorescence quantum yields J. Phys. Chem. 87 83
Ajloo D, Yoonesi B and Soleymanpour A 2010 Solvent Effect on the Reduction Potential of Anthraquinones Derivatives. The Experimental and Computational Studies Int. J. Electrochem. Sci. 5459; (b) Shaikh A M, Sharma B K and Kamble R M 2016 Electron-deficient molecules: photophysical, electrochemical and thermal investigations of naphtho[2,3-\(f\)]quinoxaline-7,12-dione derivatives Chem. Heterocycl. Comp. 52 110; (c) Shaikh A M, Sharma B K, Chacko S and Kamble R M 2016 Synthesis and optoelectronic investigations of triarylamines based on naphtho[2,3-\(f\)]quinoxaline-7,12-dione core as donor–acceptors for n-type materials RSC Adv. 6 60084
(a) Schulz G L, Kar P, Weidelener M, Vogt A, Urdanpilleta M, Lind’en M, Osteritz E M and Bäuerle A M P 2016 The influence of alkyl side chains on molecular packing and solar cell performance of dithienopyrrole-based oligothiophenes J. Mater. Chem. A 4 10514; (b) Cekli S, Winkel R W, Alarousu E, Mohammed O F and Schanze K S 2016 Triplet excited state properties in variable gap \(\pi \)-conjugated donor–acceptor–donor chromophores Chem. Sci. 7 3621
(a) Usta H, Facchetti A and Marks T J 2011 n—Channel Semiconductor Materials Design for Organic Complementary Circuits Acc. Chem. Res. 44 501; (b) Gao X and Hu Y 2014 Development of n—type organic semiconductors for thin film transistors: a viewpoint of molecular design J. Mater. Chem. C 2 3099; (c) Wang Z, Kim C, Facchetti A and Marks T J 2007 Anthracenedicarboximides as Air-Stable N-Channel Semiconductors for Thin-Film Transistors with Remarkable Current On—Off Ratios J. Am. Chem. Soc. 129 13362; (d) Jones B A, Facchetti A and Wasielewski M R 2007 Tuning Orbital Energetics in Arylene Diimide Semiconductors. Materials Design for Ambient Stability of n-Type Charge Transport J. Am. Chem. Soc. 129 15259
Tang C W and VanSlyke S A 1987 Organic Electroluminescent devices Appl. Phys. Lett. 51 913
Tonzola C J, Alam M M, Kaminsky W and Jenekhe S A 2003 New n-Type Organic Semiconductors:-Synthesis, Single Crystal Structures, Cyclic Voltammetry, Photophysics, Electron Transport, and Electroluminescence of a Series of Diphenylanthrazolines J. Am. Chem. Soc. 125 13548
Tabatake S, Naka S, Okada H, Onnagawa H, Uchida M, Nakano T and Furukawa K 2002 Low operational voltage of electroluminescent devices with a high bipolar conducting silole derivative Jpn. J. Appl. Phys. 41 6582
Fukuda T, Kanbara T, Yamamoto T, Ishikawa K, Takezoe H and Fukuda A 1996 Polyquinoxaline as an excellent electron injecting material for electroluminescent device Appl. Phys. Lett. 68 2346
(a) Brien D O, Weaver M S, Lidzey D G and Bradley D D C 1996 Use of poly(phenyl quinoxaline) as an electron transport material in polymer light-emitting diodes Appl. Phys. Lett. 69 881; (b) Cui Y, Zhang X and Jenekhe S A 1999 Thiophene-Linked Polyphenylquinoxaline. A New Electron Transport Conjugated Polymer for Electroluminescent Devices Macromolecules 32 3824
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A, T Jr, Vreven, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, AyalaP Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C and Pople J A 2004 Gaussian 03 Revision D.02 (Gaussian, Inc.: Wallingford CT,)
Zhu Y, Kulkarni A P, Wu P-T and Jenekhe S A 2008 New Ambipolar Organic Semiconductors: Synthesis, Single-Crystal Structures, Redox Properties, and Photophysics of Phenoxazine-Based Donor—Acceptor Molecules Chem. Mater. 20 4200
Yu M X, Duan J P, Lin C H, Cheng C H and Tao Y T 2002 Diaminoanthracene Derivatives as High-Performance Green Host Electroluminescent Materials Chem. Mater. 14 3958
Acknowledgements
The authors thank the Science and Engineering Research Board (SERB), New Delhi, India for financial support [SERB Scheme No. SB/EMEQ–507/2014]. BKS and AS thank the University Grant Commission, India for research fellowships. We thank the Micro-Analytical Laboratory, Department of Chemistry, University of Mumbai, Mumbai for providing instrumentation facility. We also thank the Tata Institute of Fundamental Research, Mumbai for MALDI-TOF and \(^{1}\)H–NMR facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, B.K., Shaikh, A.M., Chacko, S. et al. Synthesis and optoelectronic investigation of triarylamines based on imidazoanthraquinone as donor–acceptors for n-type materials. J Chem Sci 130, 49 (2018). https://doi.org/10.1007/s12039-018-1443-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1443-2