Abstract
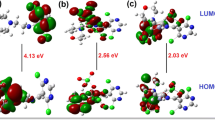
The compound N\(^\prime \)-[(Z)-furan-2-ylmethylidene]-1,3-benzothiazole-2-carbohydrazone (FMBC) and its Cu (II), Ni (II) and Zn (II) complexes were synthesized and characterized by Mass, FT-IR, NMR, elemental analysis, TGA, ESR and SEM-EDX. Based on spectro-analytical data, geometries have been assigned to the metal complexes in which the FMBC acted as bidentate chelate in its mono dissociated form (pKa = 10.56). HyperChem 7.5 software was used for quantum chemical calculations using semi-empirical method. The eigenvalues of frontier orbitals and their corresponding contour maps of HOMO and LUMO for the title compound were computed by means of single point PM3 method. DNA binding of the synthesized complexes with calf thymus DNA was studied by spectrophotometry, fluorescence and viscosity techniques. From the experimental results, it is found that the complexes bind effectively to CT-DNA through an intercalative mode. Molecular docking studies with host DNA moiety inferred corroborative results for binding affinity of the compounds in accordance with experimental data. \(\hbox {IC}_{50}\) values calculated from the cytotoxicity studies carried out on HeLa cell line by MTT assay inferred obviously enhanced activity of the metal complexes over unbound free ligand. The candidate compounds screened for antimicrobial activity against bacterial species Bacillus, Escherichia coli, Staphylococcus, Pseudomonas and fungal species Sclerotium rolfsii and Macrophomina phaseolina signified greater potency of metal complexes over free carbohydrazone ligand.
Graphical Abstract
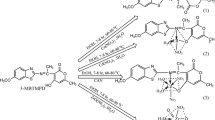
SYNOPSIS N\(^\prime \)-[(Z)-furan-2-ylmethylidene]-1,3-benzothiazole-2-carbohydrazone (FMBC) and its Cu(II), Ni(II) and Zn(II) complexes were synthesized and characterized by various spectro-analytical techniques. From the DNA binding studies, it is found that the complexes bind effectively to CT-DNA through an intercalative mode.
















Similar content being viewed by others
References
Cozzi P G 2004 Metal–Salen Schiff base complexes in catalysis: practical aspects Chem. Soc. Rev. 33 410
Ravi M, Kishan Prasad C H, Ushaiah B, Ravi Kumar E, Shyam P, Rajanna A and Sarala Devi C H 2015 A Study on Spectro-Analytical Aspects, DNA –Interaction, Photo-Cleavage, Radical Scavenging, Cytotoxic Activities, Antibacterial and Docking Properties of 3-(1-(6-methoxybenzo [d] thiazol-2-ylimino) ethyl)-6-methyl-3H-pyran-2, 4-dione and its Metal Complexes J. Fluoresc. 25 1279
Kishan Prasad C H, Ravi M, Ushaiah B, Srinu V, Ravi Kumar E and Sarala Devi C H 2016 Synthesis, characterization, DNA interactions, DNA cleavage, radical scavenging activity, antibacterial, anti-proliferative and docking studies of new transition metal complexes J. Fluoresc. 26 189
Aparup P, Soumen M, Apurba B, Soumen M, Horst P and Subal Chandra Manna 2016 RSC Adv. 6 6048
Delaney S, Pascaly M, Bhattacharya P K, Han K and Barton J K 2002 Oxidative damage by ruthenium complexes containing the dipyridophenazine ligand or its derivatives a focus on intercalation Inorg. Chem. 41 1966
Bergeron K L, Murphy E L, Olulade M, Muñoz L D, Williams J C and Almeida K H 2009 Arylphosphonium salts interact with DNA to modulate cytotoxicity Mutat. Res. 673 141
Strekowski L and Wilson B 2007 Noncovalent interactions with DNA: An overview Mutat. Res-Fund. Mol. M. 623 3
Palchaudhuri R and Hergenrother P 2007 DNA as a target for anticancer compounds: methods to determine the mode of binding and the mechanism of action J. Curr. Opin. Biotech. 18 497
Yadav P S, Dev P and Senthilkumar G P 2011 Benzothiazole Different methods of synthesis and diverse biological activities Int. J. Pharm. Sci. Res. 3 01
Akhtar T, Hameed S, Al-Masoudi N, Loddo R and Colla P L 2008 In vitro antitumor and antiviral activities of new benzothiazole and 1,3,4-oxadiazole-2-thione derivatives Acta Pharma 58 135
Bradshaw T D, Wrigley S, Shi D F, Schultz R J and Stevens M F G 19982 (4-aminophenyl) benzothiazoles: novel agents with selective profiles of in vitroantitumor activity Br. J. Cancer 77 745
Pranav P, Das S K, Shafaat K and Anand J P 2012 Evaluation of N-(6-Chloro benzothiazol-2-yl)-2-(substituted amino) Acetamide for its antibacterial activity Int. J. Pharm. Sci. Res. 3 2669
Devmurari V P, Shivanand P, Goyani M B, Nandanwar R R, Jivani N P and Perumal P 2010 Synthesis and Anticancer Activity of Some Novel 2-Substituted Benzothiazole Derivatives Int. J. Chemtech Res. 2 681
Kamal A, Reddy K S, Khan M, Shetti R, Ramaiah M, Pushpavalli S N, Srinivas C, Bhadra M P, Chourasia M, Sastry G N, Juvekar A, Zingde S and Barkume M 2010 Synthesis, DNA-binding ability and anticancer activity of benzothiazole/benzoxazolepyrrolo[2,1-c][1,4] benzodiazepine conjugates Bioorg. Med. Chem. 18 4747
Shi X H, Wang Z, Xia Y, Ye T H, Deng M, Xu Y Z, Wei Y Q and Yu L T 2012 Synthesis and biological evalution of novel benzothiazole-2-thiolderivatives as potential anticancer agent Molecule 17 3933
Suresh S H, Venkateshwara R J and Jayaveera K N 2010 Synthesis of 4-(2’-substituted benzothiazoles)-5-mercapto-3-(substituted)-1,2,4-triazole derivatives for possible antimicrobiologicalactivities Res. J. Pharma Biol. Chem. Sci. 1 635
Alang G, Kaur R, Kaur G, Singh A and Singla P 2010 Synthesis and antibacterial activity of some new benzothiazole derivatives Acta Pharm. Sci. 52 213
Imramovsky A, Pejchal V, Pankova S S, Vorcakova K, Jampilek J, Vanco J, Simunek P, Kralovec K, Bruckova L, Mandikova J and Trejtnar F 2013 Synthesis and in vitro evaluation of new derivatives of 2-substituted-6-fluorobenzo[d] thiazoles as cholinesterase inhibitors Bioorg. Med. Chem. 21 735
Sathe B S, Jayachandran E, Jagtap V A and Sreenivasa G M 2011 Anthelmintic activity of newly synthesized moieties of fluorobenzothiazole Schiff’s bases Res. J. Pharma Biol. Chem. Sci. 2 510
Pattan S, Suresh C, Pujar V, Reddy V, Rasal V and Koti B 2005 Synthesis and antidiabetic activity of 2-amino [5(4-sulphonyl benzylidine)-2,4-thiazolidinenone]-7-chloro-6-fluro benzothiazole Indian J. Chem. 44B 2404
Narsimha N, Ranjithreddy P, Jaheer M D, Aparna B and Saraladevi C H 2015 Synthesis, Characterization and Biological Studies of Novel (16Z)-1-ethyl-1, 4-dihydro-N’-(1-(3, 4- dihydro-6-methyl-2, 4-dioxo-2H-pyran-3-yl)ethylidene)-7-methyl-4-oxo-1, 8-naphthyridine 3- carbohydrazide) and its Cu (II), Ni (II) and Co (II) Complexes Int. J. Res. Pharm. Chem. 5 615
Aparna A V, Sudeepa K, Raghavaiah P and Sarala Devi C H 2013 Spectro-analytical-ray diffraction and computational studies on N-[(2-hydroxyphenyl) methylidene] acetohydrazide and its copper complex J. Indian Chem. Soc. 90 33
Hyperchem 2003 Hypercube, Inc.
Searle M S, Maynard A J and Williams H E 2003 DNA recognition by the anthracycline antibiotic respinomycin D: NMR structure of the intercalation complex with the (AGACGTCT)2 Org. Biomol. Chem. 1 60
Michel F and Sanner 1999 Python: A Programming Language for Software Integration and Development J. Mol. Graphics Mod. 17 57
Campaingne E 1958 Reaction of Diethyl Oxalate with Some ortho-Substituted Anilines J. Org. Chem. 23 1344
Campainge E, Thompson R L and Vanwerth J E 1959 Some Heterocyclic Aldehyde Thiosemicarbazones Possing Anti-viral Activity J. Med. Chem. 1 577
Shakir M, Summaiya H, FazleAlam M D and Hina Y 2016 Molecular hybridization approach of bio-potent Cu(II)/Zn(II) complexes derived from N, O donor bidentate imine scaffolds: Synthesis, spectral, human serum albumin binding, antioxidant and antibacterial studies J. Photochem. Photobiol. 165 96
Nagesh G Y and Mruthyunjaya swamy B H M 2015 Synthesis, characterization and biological relevance of some metal (II) complexes with oxygen, nitrogen and oxygen (ONO) donor Schiff base ligand derived from thiazole and 2-hydroxy-1-naphthaldehyde J. Mol. Struct. 1085 198
Nakamoto K 1997 Infrared and Raman Spectra of Inorganic and Coordination Compounds \(5^{\rm th}\) edn. (New York: Wiley-Interscience)
Bellamy L J 1980 The Infrared Spectra of Complex Molecules \(2^{\rm nd}\) ed. (London: Chapmann and Hall)
Naeimi H and Moradian M 2010 Synthesis and characterization of nitro-Schiff bases derived from 5-nitro-salicylaldehyde and various diamines and their complexes of Co(II) J. Coord. Chem. 63 156
Kavitha P et al 2013 Synthesis, structural characterization, fluorescence, antimicrobial, antioxidant and DNA cleavage studies of Cu(II) complexes of formyl chromone Schiff bases Spectrochim. Acta A 102 159
Sebastian M, Arun V, Robinson P P, Varghese A A, Abraham R, Suresh E and Yusuff K K M 2010 Synthesis, structural characterization and catalytic activity study of Mn (II), Fe(III), Ni(II), Cu(II) and Zn(II) complexes of quinoxaline-2-carboxalidine-2-amino-5-methylphenol: Crystal structure of the nickel(II) complex Polyhedron 29 3014
Tweedy B G 1964 Plant extracts with metal ions as potential antimicrobial agents Phytopathology 55 910
Lu J, Sun Q, Li J L, Gu W, Tianab J L, Liuab X and Yan S P 2013 Synthesis, characterization, and DNA-binding of two new Cd(II) complexes with 8-[(2-pyridylmethyl)amino]-quinoline J. Coord. Chem. 66 3280
Liu H K and Sadler P J 2011 Metal complexes as DNA intercalators Acc. Chem. Res. 44 349
Stern O and Volmer M 1919 Über die Abklingzeit der Fluoreszenz Physik. Zeitschr. 20 183
Tabrizi L, McArdle P, Erxleben A and Chiniforoshan H 2015 Nickel (II) and cobalt (II) complexes of lidocaine Synthesis, structure and comparative in vitro evaluations of biological perspectives Eur. J. Med. Chem. 103 516
Tarui M, Doi M, Ishida T, Inoue M, Nakaike S and Kitamura K 1994 DNA-binding characterization of a novel anti-tumourbenzo[a]- phenazine derivative NC-182 spectroscopic and viscometric studies Biochem. J. 304 271
Guo H, Lu J, Ruan Z, Zhang Y, Liu Y, Zang L, Jiang J and Huang J 2012 Synthesis, DNA-binding, cytotoxicity, and cleavage studies of unsymmetrical oxovanadium complexes J. Coord. Chem. 65 191
Acknowledgements
We express our gratitude to Department of Chemistry, Nizam College, Basheerbagh, Osmania University for providing lab facilities for synthesis and Department of Chemistry, Osmania University for providing Instrumentation Lab Facilities, and the Central Facilities for Research & Development (CFRD) for recording IR and NMR Spectra. We acknowledge Sophisticated Analytical Instrument Facility (SAIF), IIT Bombay for recording ESR spectra and Biogenics Research and Training Center, Hubli, Karnataka for cytotoxicity studies. We are deeply thankful for the generous funding extended from UGC-FAR and DST-PURSE Projects.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sudeepa, K., Narsimha, N., Aparna, B. et al. Synthesis, spectral characterization, antimicrobial, DNA interactions and molecular modeling studies of metal complexes of 1, 3-benzothiazole carbohydrazone. J Chem Sci 130, 52 (2018). https://doi.org/10.1007/s12039-018-1437-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1437-0