Abstract
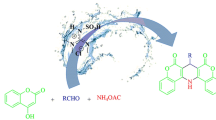
Rhodanine derivatives are highly valuable heterocycles in drug discovery. Here, we developed aldol reaction of N-substituted rhodanines and aromatic aldehydes on water. The reaction was performed at room temperature affording the products in good to high yield. This synthetic protocol uses simple experimental procedures, catalyst-free, and avoids the use of highly toxic solvents.
Graphical Abstract
SYNOPSIS Aldol reaction of N-substituted rhodanines and aromatic aldehydes on water at room temperature has been described. This protocol is simple, catalyst-free, and avoids the use of highly toxic solvents.




Similar content being viewed by others
References
Anastas P T and Warner J C 2000 Green Chemistry: Theory and Practice (Oxford: Oxford University Press)
(a) Mehra M K, Tantak M P, Arun V, Kumar I and Kumar D 2017 Metal-free regioselective formation of C-N and C-O bonds with the utilization of diaryliodonium salts in water: Facile synthesis of \(N\)-arylquinolones and aryloxyquinolines Org. Biomol. Chem. 15 4956; (b) Lindstrom U M 2002 Stereoselective organic reactions in water Chem. Rev. 102 2751
Chanda A and Fokin V V 2009 Organic synthesis “on water” Chem. Rev. 109 725
Irvine M W, Patrick G L, Kewney J, Hastings S F and Mackenzie S J 2008 Rhodanine derivatives as novel inhibitors of PDE4 Bioorg. Med. Chem. Lett. 18 2032
Silva A A R, Góes A J S, Lima W T and Maia M B S 2003 Antiedematogenic activity of two thiazolidine derivatives: \(N\)-Tryptophyl-5-(3,5-di-tert-butyl-4-hydroxybenzylidene) Rhodanine (GS26) and \(N\)-Tryptophyl-5-(3,5-di-tert-butyl-4-hydroxybenzylidene)-2,4-thiazolidinedione (GS28) Chem. Pharm. Bull. 51 1351
Inamori Y, Okamoto Y, Takegawa Y, Tsujibo H, Sakagami Y, Kumeda Y, Shibata M and Numata A 1998 Insecticidal and antifungal activities of aminorhodanine derivatives Biosci. Biotechnol. Biochem. 62 1025
Terashima H, Hama K, Yamamoto R, Tsuboshima M, Kikkawa R, Hantanaka I and Shigeta Y 1984 Effects of a new aldose reductase inhibitor on various tissue in vitro J. Pharmacol. Exp. Ther. 229 226
Villain-Guillot P, Gualtieri M, Bastide L, Roquet F, Martinez J, Amblard M, Pugniere M and Leonetti J-P 2007 Structure-activity relationships of phenyl-furanyl-rhodanines as inhibitors of RNA polymerase with antibacterial activity on biofilms J. Med. Chem. 50 4195
Dayam R, Sanchez T, Clement O, Shoemaker R, Sei S and Neamati N 2005 \(\beta \)-Diketo acid pharmacophore hypothesis. 1. Discovery of a novel class of HIV-1 integrase inhibitors J. Med. Chem. 48 111
Sing W T, Lee C L, Yeo S L, Lim S P and Sim M M 2001 Arylalkylidene rhodanine with bulky and hydrophobic functional group as selective HCV NS3 protease inhibitor Bioorg. Med. Chem. Lett. 11 91
Grant E B, Guiadeen D, Baum E Z, Foleno B D, Jin H, Montenegro D A, Nelson E A, Bush K and Hlasta D J 2000 The synthesis and SAR of rhodanines as novel class C \(\beta \)-lactamase inhibitors Bioorg. Med. Chem. Lett. 10 2179
Talele T T, Arora P, Kulkarni S S, Patel M R, Singh S, Chudayeu M and Basu N K 2010 Structure-based virtual screening, synthesis and SAR of novel inhibitors of hepatitis C virus NS5B polymerase Bioorg. Med. Chem. Lett. 18 4630
Whitesitt C A, Simon R L, Reel J K, Sigmund S K, Phillips M L, Shadle J K, Heinz L J, Koppel G A, Hunden D C, Lifer S L, Berry D, Ray J, Little S P, Liu X, Marshall W S and Panetta J A 1996 Synthesis and structure-activity relationships of benzophenones as inhibitors of cathepsin D Bioorg. Med. Chem. Lett. 6 2157
(a) Rostamnia S, Zeynizadeh B, Doustkhah E, Baghban A and Aghbash K O 2015 The use of \(\kappa \)-carrageenan/\({\text{Fe}}_{3}{\text{ O }}_{4}\) nanocomposite as a nanomagnetic catalyst for clean synthesis of rhodanines Catal. Commun. 68 77; (b) Rostamnia S, Doustkhah E and Nuri A 2013 Hexafluoroisopropanol dispersed into the nanoporous SBA-15 (HFIP/SBA-15) as a rapid, metal-free, highly reusable and suitable combined catalyst for domino cyclization process in chemoselective preparation of alkyl rhodanines J. Fluor. Chem. 153 1; (c) Alizadeh A, Rostamnia S, Zohreh N and Hosseinpour R 2009 A simple and effective approach to the synthesis of rhodanine derivatives via three-component reactions in water Tetrahedron Lett. 50 1533; (d) Rostamnia S 2011 A rapid, catalyst-free, three-component synthesis of rhodanines in water using ultrasound Synthesis 2011 3080; (e) Metwally M A, Etman H A, Keshk E M and Fekry A 2006 Thiazolidin-5-ones: Synthesis and reactions Phosphorus Sulfur 181 1039; (f) Mulay A, Mangesh G and Nikalje A P 2009 Exploring potential of 4-thiazolidinone: A brief review Int. J. Pharm. Sci. 1 46
List B 2002 Proline-catalyzed asymmetric reactions Tetrahedron 58 5573
Schetter B and Mahrwald R 2006 Modern aldol methods for the total synthesis of polyketides Angew. Chem. Int. Edit. 45 7506
Trost B M and Brindle C S 2010 The direct catalytic asymmetric aldol reaction Chem. Soc. Rev. 39 1600
(a) Rohr K and Marhrwald R 2008 Catalyst-free aldol additions of 1,3-dicarbonyl compounds Adv. Synth. Catal. 350 2877; (b) Curtimgrt C, Battistini L, Zanardi F, Rassu G, Zambrano V, Pinna L and Casiraghi G 2010 Uncatalyzed, diastereoselective vinylogous Mukaiyama aldol reactions on aqueous media: Pyrrole vs furan 2-silyloxy dienes J. Org. Chem. 75 8681; (c) Sartori A, Dell’Amico L, Curti C, Battistini L, Pelosi G, Rassu G, Casiraghi G and Zanardi F 2011 Aqueous and solvent-free uncatalyzed three-component vinylogous mukaiyama-mannich reactions of pyrrole-based silyl dienolates Adv. Synth. Catal. 353 3278; (d) Paladhi S, Bhati M, Panda D and Dash J 2014 thiazolidinedione-isatin conjugates via an uncatalyzed diastereoselective aldol reaction on water J. Org. Chem. 79 1473; (e) Paladhi S, Chauhan A, Dhara K, Tiwari A K and Dash J 2012 An uncatalyzed aldol reaction of thiazolidinediones Green Chem. 14 2990
Lesyk R B and Zimenkovsky B S 2004 4-Thiazolidones: centenarian history, current status and perspectives for modern organic and medicinal chemistry Curr. Org. Chem. 8 1547
Acknowledgements
The authors acknowledge the support of this research by the Science and Engineering Research Board (SERB) (SB/FT/CS-170/2013), New Delhi, India. Authors are also thankful to Central Instruments Facility (CIF), IIT Guwahati, for spectral recording.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Devi, N.S., Devi, N. Catalyst-free aldol reaction of N-substituted rhodanines on aqueous media. J Chem Sci 130, 18 (2018). https://doi.org/10.1007/s12039-018-1419-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1419-2