Abstract
In the present article a group theoretical approach has been used to explain different electron-count rules for aromaticity. A general group theoretical method is presented to derive and unite the different electron count rules (Hückel, Baird, Möbius, and Spherical aromaticity). It is shown that continuous groups play important role in understanding of these electron count rules.
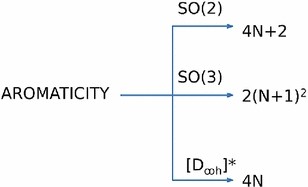
Graphical Abstract
SYNOPSIS Group theory has been used to investigate the origin of electron-count rules. Present work sucessfully classified the different types of aromaticity in terms of irrudicible representations of continuous groups.






Similar content being viewed by others
References
Hofmann A W 1856 On insolinic acid Proc. R. Soc. Lond. 8 1
Kekulé A 1865 Sur la constitution des substances aromatiques Bull. Soc. Chim. Fr. (Paris) 3 98
Clayden J, Greeves N, Warren S and Wothers P 2001 Organic Chemistry (New York: Oxford University Press)
Schleyer P von R 2011 Introduction: Aromaticity Chem. Rev. 101 1115
Garrat P J 1986 Aromaticity (New York: Wiley)
Boldyrev A I and Wang L S 2005 All-metal aromaticity and antiaromaticity Chem. Rev. 105 3716
King R B 2001 Three-dimensional aromaticity in polyhedral boranes and related molecules Chem. Rev. 101 1119
Chen Z and King R B 2005 Spherical aromaticity: recent work on fullerenes, polyhedral boranes, and related structures Chem. Rev. 105 3613
Bühl M and Hirsch A 2001 Spherical aromaticity of fullerenes Chem. Rev. 101 1153
Garcia-Borràs M, Osuna S, Luis J M, Swart M and Solà M 2014 The role of aromaticity in determining the molecular structure and reactivity of (endohedral metallo) fullerenes Chem. Soc. Rev. 43 5089
Ottosson H 2012 Organic photochemistry: Exciting excited-state aromaticity Nat. Chem. 4 969
Datta A and Pati S K 2006 Stability of cyclic \(({H_2O})_n\) clusters within molecular solids: Role of aromaticity Int. J. Quantum Chem. 106 1697
Zubarev D Y, Averkiev B B, Zhai H J, Wang L S and Boldyrev A I 2008 Aromaticity and antiaromaticity in transition-metal systems Phys. Chem. Chem. Phys. 10 257
Yu X, Oganov A R, Popov I A and Boldyrev A I 2016 d-AO spherical aromaticity in \(\text{Ce}_6\text{ O }_8\) J. Comput. Chem. 37 103
Heilbronner E 1964 Hückel molecular orbitals of Möbius-type conformations of annulenes Tetrahedron Lett. 5 1923
Zimmerman H E 1966 On molecular orbital correlation diagrams, the occurrence of Möbius systems in cyclization reactions, and factors controlling ground-and excited-state reactions J. Am. Chem. Soc. 88 1564
Zimmerman H E 1971 Möbius-Hückel concept in organic chemistry. Application of organic molecules and reactions Acc. Chem. Res. 4 272
Rzepa H S 2005 Möbius aromaticity and delocalization Chem. Rev. 105 3697
Yoon Z S, Osuka A and Kim D 2009 Möbius aromaticity and antiaromaticity in expanded porphyrins Nat. Chem. 1 113
Cyranski M K, Krygowski T M, Katritzky A R and Schleyer P v R 2002 To what extent can aromaticity be defined uniquely? J. Org. Chem. 67 1333
Katritzky A R, Karelson M, Sild S, Krygowski T M and Jug K 1998 Aromaticity as a quantitative concept. 7. Aromaticity reaffirmed as a multidimensional characteristic J. Org. Chem. 63 5228
Krygowski T M, Szatylowicz H, Stasyuk O A, Dominikowska J and Palusiak M 2014 Aromaticity from the viewpoint of molecular geometry: Application to planar systems Chem. Rev. 114 6383
Noorizadeh S and Shakerzadeh E. 2010 Shannon entropy as a new measure of aromaticity, Shannon aromaticity , Shannon aromaticity Phys. Chem. Chem. Phys. 12 4742
Chen Z, Wannere C S, Corminboeuf C, Puchta R and Schleyer P v R 2005 Nucleus-independent chemical shifts (NICS) as an aromaticity criterion Chem. Rev. 105 3842
Matito E, Duran M and Sola M 2005 The aromatic fluctuation index (FLU): A new aromaticity index based on electron delocalization J. Chem. Phys. 122 014109
Katritzky A R, Jug K and Oniciu D C 2001 Quantitative measures of aromaticity for mono-, bi-, and tricyclic penta-and hexaatomic heteroaromatic ring systems and their interrelationships Chem. Rev. 101 1421
Feixas F, Matito E, Poater J and Solà M 2016 In Applications of Topological Methods in Molecular Chemistry R Chauvin, C Lepetit, B Silvi and E Alikhani (Eds.) (Switzerland: Springer International Publishing) p. 321
Hirsch A, Chen Z and Jiao H 2000 Spherical aromaticity in Ih symmetrical fullerenes: The \(2(N+ 1){^2}\) rule Angew. Chem. Int. Edit. 39 3915
Baird N C 1972 Quantum organic photochemistry. II. Resonance and aromaticity in the lowest 3. pi.. pi.* state of cyclic hydrocarbons J. Am. Chem. Soc. 94 4941
Poater J and Solà M 2011 Open-shell spherical aromaticity: The \(2N^2+ 2N+ 1\) (with \(S= N+ {1/2}\)) rule Chem. Commun. 47 11647
Dewar M J S 1966 A molecular orbital theory of organic chemistry VIII: Aromaticity and electrocyclic reactions Tetrahedron Suppl 22 (Suppl. 8) 75
Miliordos E 2010 Hückel versus Möbius aromaticity: The particle in a cylinder versus a Möbius strip Phys. Rev. A 82 062118
Miliordos E 2011 Particle in a Möbius wire and half-integer orbital angular momentum Phys. Rev. A 83 062107
McKee W C, Wu I.Judy, Rzepa H S. and Schleyer P v R 2013 A Hückel theory perspective on Möbius aromaticity Organ. Lett. 15 3432
Rubin M A and Ordónez C R 1984 Eigenvalues and degeneracies for n-dimensional tensor spherical harmonics J. Math. Phys. 25 2888
Poater J, Solà M, Viñas C and Teixidor F 2014 \(\pi \) Aromaticity and Three-Dimensional Aromaticity: Two sides of the Same Coin? Angew. Chem. Int. Edit. 53 12191
Wade K 1971 The structural significance of the number of skeletal bonding electron-pairs in carboranes, the higher boranes and borane anions, and various transition-metal carbonyl cluster compounds J. Chem. Soc. D 792
Mingos D M P 1972 A general theory for cluster and ring compounds of the main group and transition elements Nat. Phys. Sci. 236 99
Fowler P W and Rzepa H S 2006 Aromaticity rules for cycles with arbitrary numbers of half-twists Phys. Chem. Chem. Phys. 8 1775
Goldstein M and Hoffmann R 1971 Symmetry, topology, and aromaticity J. Am. Chem. Soc. 93 6193
Shainyan B A 2011 Electron-counting rules, three-dimensional aromaticity, and the boundaries of the Periodic Table J. Phys. Org. Chem. 24 619
Pham H T, Duong L V and Nguyen M T 2014 Electronic structure and chemical bonding in the double ring tubular boron clusters J. Phys. Chem. C 118 24181
Van Duong L, Pham H T, Tam N M and Nguyen M T 2014 A particle on a hollow cylinder: the triple ring tubular cluster B\({_{27}}^+\) Phys. Chem. Chem. Phys. 16 19470
Tai T B, Ceulemans A and Nguyen M T 2012 Disk aromaticity of the planar and fluxional anionic boron clusters \(B{{_{20}}{^{-/2-}}}\) Chem. Eur. J. 18 4510
Tai T B, Havenith R W, Teunissen J L, Dok A R, Hallaert S D, Nguyen M T and Ceulemans A 2013 Particle on a boron disk: Ring currents and disk aromaticity in B\({_{20}}^{2-}\) Inorg. Chem. 52 10595
Tai T B, Van Duong L, Pham H T, Mai D T T and Nguyen M T 2014 A disk-aromatic bowl cluster B Tai T B, Van Duong L, Pham H T, Mai D T T and Nguyen M T 2014 A disk-aromatic bowl cluster B\(_30\): toward formation of boron buckyballs Chem. Commun. 50 1558
Alexandrova A N, Boldyrev A I, Zhai H J and Wang L-S 2006 All-boron aromatic clusters as potential new inorganic ligands and building blocks in chemistry Coord. Chem. Rev. 250 2811
Herges R 2006 Topology in chemistry: Designing Möbius molecules Chem. Rev. 106 4820
Tanaka Y, Saito S, Mori S, Aratani N, Shinokubo H, Shibata N, Higuchi Y, Yoon Z S, Kim K S and Noh S B 2008 Metalation of expanded porphyrins: A chemical trigger used to produce molecular twisting and Möbius aromaticity Angew. Chem. 120 693
Herzberg G 1966 Electronic Spectra of Polyatomic Molecules (New York: Van Nostrand)
Shainyan B. 2006 Rules for counting electrons and three-dimensional aromaticity Russ. J. Organ. Chem. 42 304
El Bakouri O, Duran M, Poater J, Feixas F and Solà M 2016 Octahedral aromaticity in \(^{2S+ 1}A_{1g}X{{_6}{^q}}\) clusters (X= Li–C and Be–Si, S= 0–3, and q= -2 to +4) Phys. Chem. Chem. Phys. 18 11700
Acknowledgements
Author acknowledges the financial support from SERB-DST, Govt. of India through the Project No. ECR/2016/000279.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, P. Continuous group and electron-count rules in aromaticity. J Chem Sci 130, 17 (2018). https://doi.org/10.1007/s12039-017-1417-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-017-1417-9