Abstract
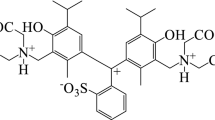
The acid-base properties of the Patented Blue V dye were studied by spectrophotometry and tristimulus colourimetry. The mechanism of protonation of Patented Blue V has been investigated with semi-empirical and DFT methods. The quantum chemical calculations of total energy defined the most stable isomer for each protonated form in water solution. In addition to thermodynamic parameters, the condensed Fukui function and molecular electrostatic potential were successfully used as reactivity descriptors for the determination of the most favorable site for protonation. Moreover, for the explanation of the structure of the most deprotonated form of the dye in highly basic medium, the weak intramolecular interactions were investigated with the reduced density gradient function. The semi-empirical calculations of absorbance spectra explained the changing of the colour of the dye for the different protonated states. It has been shown that the dominant form of the Patented Blue V was the electro-neutral form, and the molar absorptivity (\(\upvarepsilon _{639}=1.06\times 10^{5}\,\hbox {dm}^{3}\cdot \hbox {mol}^{-1}\cdot \hbox {cm}^{-1})\) increases with the increase of the dielectric permittivity of the solvent. The replacement of polar solvents by less polar ones is causing a bathochromic shift of the absorption band of the dye, the value of which is correlated with the value of the Hansen parameter.
Graphical Abstract
SYNOPSIS The acid-base properties of the Patented blue V dye were studied by spectrophotometry and tristimulus colourimetry. The mechanism of protonation of Patented Blue V has been investigated with semi-empirical and DFT methods. The ionization constants of the dye functional groups were determined and the most probable protonation/deprotonation scheme was proposed.










Similar content being viewed by others
References
Ceyhan B M, Gultekin F, Doguc D K and Kulac E 2013 Effects of maternally exposed coloring food additives on receptor expressions related to learning and memory in rats Food Chem. Toxicol. 56 145
Zevatskii Yu E, Samoilov D V and Mchedlov–Petrosyan N O 2009 Contemporary methods for the experimental determination of dissociation constants of organic acids in solutions Russ. J. Gen. Chem. 79 1859
Zevatski Yu E and Samoilov D V 2011 Modern methods for estimation of ionization constants of organic compounds in solution Russ. J. Org. Chem. 47 1445
Sovyn O R and Patsay I O 2012 Computer program “SpectroCalc-H5A” for spectrophotometric determination of acid dissociation constants Methods Objects Chem. Anal. 7 74 (in Ukrainian)
Ivanov V M, Monogarova O V and Oskolok K V 2015 Capabilities and prospects of the development of a chromaticity method in analytical chemistry J. Anal. Chem. 70 1165
Chebotarev A N and Snigur D V 2015 Study of the acid-base properties of quercetin in aqueous solutions by colour measurements J. Anal. Chem. 70 55
Chebotarev A N and Snigur D V 2016 Study of the acid-base properties of morin by tristimulus colorimetry Russ. J. Gen. Chem. 86 815
Chebotarev A N, Snigur D V, Zhukova Yu P, Bevziuk K V, Studenyak Ya I and Bazel Ya R 2017 Tristimulus colourimetric and spectrophotometric study of the state of 4-hydroxystyryl dyes in aqueous solutions Russ. J. Gen. Chem. 87 196
Stewart J J P 2013 Optimization of parameters for semiempirical methods VI: more modifications to the NDDO approximations and re-optimization of parameters J. Mol. Model. 19 1
Bevziuk K, Chebotarev A, Snigur D, Bazel Ya, Fizer M and Sidey V 2017 Spectrophotometric and theoretical studies of the protonation of Allura Red AC and Ponceau 4R J. Mol. Struct. 1144 216
Fizer M, Sukharev S, Slivka M, Mariychuk R and Lendel V 2016 Preparation of bisthiourea and 5-Amino-4-benzoyl-1,2,4-triazol-3-thione complexes of Copper (II), Nickel and Zinc and their biological evolution J. Organomet. Chem. 804 6
Fizer M, Sidey V, Tupys A, Ostapiuk Y, Tymoshuk O and Bazel Ya 2017 On the structure of transition metals complexes with the new tridentate dye of thiazole series: theoretical and experimental studies J. Mol. Struct. 1149 669
Klamt A, Moya C and Palomar J 2015 A comprehensive comparison of the IEFPCM and SS(V)PE continuum solvation methods with the COSMO approach J. Chem. Theory Comput. 11 4220
Grimme S 2006 Semiempirical GGA-type density functional constructed with a long-range dispersion correction J. Comput. Chem. 27 1787
Grimme S, Antony J, Ehrlich S and Krieg H 2010 A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu J. Chem. Phys. 132 154104
Grimme S, Ehrlich S and Goerigk L 2011 Effect of the damping function in dispersion corrected density functional theory J. Comput. Chem. 32 1456
Burns L A, Vazquez-Mayagoitia A, Sumpter B G and Sherrill C D 2011 Density-functional approaches to noncovalent interactions: a comparison of dispersion corrections (DFT-D), exchange-hole dipole moment (XDM) theory, and specialized functionals J. Chem. Phys. 134 084107
Tao J, Perdew J P, Staroverov V N and Scuseria G E 2003 Climbing the density functional ladder: nonempirical meta–generalized gradient approximation designed for molecules and solids Phys. Rev. Lett. 91 146401
Medvedev M G, Bushmarinov I S, Sun J, Perdew J P and Lyssenko K A 2017 Density functional theory is straying from the path toward the exact functional Science 355 49
Riley K E, Tran K, Lane P, Murray J S and Politzer P 2016 Comparative analysis of electrostatic potential maxima and minima on molecular surfaces, as determined by three methods and a variety of basis sets J. Comput. Sci. 17 273
Cao J, Ren Q, Chen F and Lu T 2015 Comparative study on the methods for predicting the reactive site of nucleophilic reaction Sci. China Chem. 58 1845
Stewart J J P MOPAC 2016 Version 17.173W Stewart Computational Chemistry. Accessed on: http://OpenMOPAC.net (accessed on 12 June 2017)
Neese F 2012 The ORCA program system WIREs Comput. Mol. Sci. 2 73
Laikov D N 1997 Fast evaluation of density functional exchange-correlation terms using the expansion of the electron density in auxiliary basis sets Chem. Phys. Lett. 281 151
Johnson E R, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen A J and Yang W 2010 Revealing Noncovalent Interactions J. Am. Chem. Soc. 132 6498
Lu T and Chen F 2012 Multiwfn: A multifunctional wavefunction analyzer J. Comput. Chem. 33 580
Hanwell M D, Curtis D E, Lonie D C, Vandermeersch T, Zurek E and Hutchison G R 2012 Avogadro: an advanced semantic chemical editor, visualization, and analysis platform J. Cheminform. 4 1
Allouche A R 2011 Gabedit - A graphical user interface for computational chemistry softwares J. Comput. Chem. 32 174
Humphrey W, Dalke A and Schulten K 1996 VMD: Visual molecular dynamics J. Mol. Graph. 14 33
Lešková M, Bazel Ya, Torok M and Studenyak Ya 2013 Structure and properties of 2-[(E)-2-(4-dipropylaminophenyl)-1-ethenyl]-1,3,3-trimethyl-3H-indolium chloride Chem. Pap. 67 415
Billes F, Szabo A and Studenyak Ya 2011 Vibrational spectroscopic study on 2-[2-(4-dipropylamino-phenyl)-vinyl]-1,3,3-trimethyl-3H-indolium chloride Spectrochim. Acta A 78 967
Parr R G and Yang W 1989 In Density Functional Theory of Atoms and Molecules (New York, Oxford: Oxford University Press) p. 333
Hansen C M 2007 In Solubility Parameters: A User’s Handbook (Boca Raton: CRC Press Taylor & Francis Group) p. 521
Thompson M A and Zerner M C 1991 A Theoretical examination of the electronic structure and spectroscopy of the photosynthetic reaction center from rhodopseudomonas viridis J. Am. Chem. Soc. 113 8210
Brückner C and Engels B 2015 Benchmarking ground-state geometries and vertical excitation energies of a selection of p-type semiconducting molecules with different polarity J. Phys. Chem. A 119 12876
Acknowledgements
27 structures at different protonated states are shown with the difference in energy between the current form and the most stable form. Data for each state is available as Supplementary Information for this article at www.ias.ac.in/chemsci.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bevziuk, K., Chebotarev, A., Fizer, M. et al. Protonation of Patented Blue V in aqueous solutions: theoretical and experimental studies. J Chem Sci 130, 12 (2018). https://doi.org/10.1007/s12039-017-1411-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-017-1411-2