Abstract
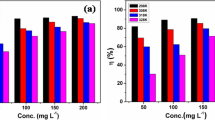
Three pyridine-based Schiff bases namely \(\hbox {N}^{2}\hbox {,N}^{6}\)-bis(4-methylbenzylidene)pyridine-2,6-diamine (DAP-1), \(\hbox {N}^{2}\hbox {,N}^{6}\)-dibenzylidenepyridine-2,6-diamine (DAP-2) and \(\hbox {N}^{2}\hbox {,N}^{6}\)-bis(4-nitrobenzylidene)pyridine-2,6-diamine (DAP-3) were synthesized, characterized, and their corrosion inhibition performance was studied on mild steel (MS) in 1 M hydrochloric acid solution using electrochemical experiments and theoretical study. The results showed that all the three DAPs act as mixed type corrosion inhibitors, and are adsorbed on MS surface by following Langmuir adsorption isotherm. The methyl-substituted DAP-1 showed maximum inhibition effiency of 98.5% at \(40 \hbox { mgL}^{-1}\). The formation of inhibitor film on MS surface was confirmed by SEM and AFM. Quantum chemical calculations and Monte Carlo simulations were used to understand metal-inhibitor interaction and orientation of adsorption of DAP molecules. A good correlation was observed between theoretical and experimental results.
Graphical Abstract
Synopsis Three pyridine-based Schiff bases were synthesized, characterized, and their corrosion inhibition performance was studied on mild steel in 1 M HCl solution. Results of gravimetric measurements, potentiodynamic polarization, EIS, and SEM, AFM image analyses are reported. The inhibitor molecules follow Langmuir isotherm and showed mixed-type behavior. Experimental results were supported by DFT calculations and Monte Carlo simulations.










Similar content being viewed by others
References
Hosseini M G, Ehteshamzadeh M and Shahrabi T 2007 Protection of mild steel corrosion with Schiff bases in 0.5M \({\rm H}_{2}{\rm SO}_{4 }\) solution Electrochim. Acta 52 3680
Behpour M, Ghoreishi S M, Niasar AG, Soltani N and Niasari M S 2009 The inhibition of mild steel corrosion in hydrochloric acid media by two Schiff base compounds J. Mater. Sci. 44 2444
Fan H B, Fu C Y, Wang H L, Guo X P and Zheng J S 2002 Inhibition of corrosion of mild steel by sodium n,n-diethyl dithiocarbamate in hydrochloric acid solution Brit. Corros. J. 37 122
Bose D, Banerjee J, Rahaman S K H, Mostafa G, Fun H K, Bailey W R D, Zaworotko M J and Ghosh B K 2004 Polymeric end-to-end bibridged cadmium(II)thiocyanates containing monodentate and bidentate N-donor organic blockers: supramolecularsynthons based on \(\pi \)–\(\pi \) and/or \(\text{ C }\)–\(\text{ H }\cdots \pi \) interactions Polyhedron 23 2045
Abd El-Maksoud S A 2008 The Effect of Organic Compounds on the Electrochemical Behaviour of Steel in Acidic Media A review Int. J. Electrochem. Sci. 3 528
Ansari K R, Quraishi M A and Singh A 2015 Pyridine derivatives as corrosion inhibitors for N80 steel in 15% HCl: Electrochemical, surface and quantum chemical studies Measurement 76 136
Douadi T, Hamani H, Chafaa S and Noaim M A 2015 Corrosion inhibition of mild steel by two new S-heterocyclic compounds in 1 M HCl: Experimental and computational study Corros. Sci. 94 21
Zarrok H, Oudda H, Zarrouk A, Salghi R, Hammouti B and Bouachrine M 2011 Weight Loss Measurement and Theoretical Study of New Pyridazine Compound as Corrosion Inhibitor for C38 Steel in Hydrochloric Acid Solution D. Pharma Chem. 3 576
Popova A, Christov M, Raicheva S and Sokolova E 2004 Adsorption andinhibitive properties of benzimidazole derivatives in acid mildsteel corrosion Corros. Sci. 46 1333
Bentiss F, Lebrini M, Vezin H, Chai F, Traisne M and Lagrenée M 2009 Enhanced corrosion resistance of carbon steel in normal sulfuric acid medium by some macrocyclic polyether compounds containing a 1,3,4-thiadiazole moiety AC impedance and computational studies Corros. Sci. 51 2165
Pardavé P M, Romo R M, Hernández H H, Quijano M A A, Likhanova N V, Uruchurtu J and Juárez-García J M 2012 Influence of the alkyl chain length of 2 amino 5 alkyl 1,3,4 thiadiazole compounds on the corrosion inhibition of steel immersed in sulfuric acid solutions Corros. Sci. 54 231
Obot I B, Obi-Egbedi N O and Umoren S A 2009 Ginseng root: a new efficient and effective ecofriendly corrosion inhibitor for aluminum alloy of type AA 1060 in hydrochloric acid solution Int. J. Electrochem. Sci. 4 863
Quraishi M A and Sardar R 2003 Hector bases–a new class of heterocyclic corrosion inhibitors for mild steel in acid solutions J. Appl. Electrochem. Soc. 33 1163
Khaled K F 2010 Experimental and molecular dynamics study on the inhibition performance of some nitrogen containing compounds for iron corrosion Mater. Chem. Phys. 124 760
Nataraja S E, Venkatesha T V, Tandon H C and Shylesha B S 2011 Quantum chemical and experimental characterization of the effect of ziprasidone on the corrosion inhibition of steel in acid media Corros. Sci. 53 4109
Silva C M, Silva D N, Modolo L V, Alves R B, Resende M A, Martins C V B and Fatima A 2011 Schiff bases: A short review of their antimicrobial activities J. Adv. Res. 2 1
Sılkul P, Ozkınal S, OztürkZ, Asan A and Köse A 2016 Synthesis of novel Schiff Bases containing acryloyl moiety and the investigation of spectroscopic and electrochemical properties J. Mol. Struct. 1116 72
Small B L, Brookhart M and Bennett A M A 1998 Highly Active Iron and Cobalt Catalysts for the Polymerization of Ethylene J. Am. Chem. Soc. 120 4049
Ulusoy M, Birel O, Sahin O, Buyukgungor O and Cetinkya B 2012 Structural, spectral, electrochemical and catalytic reactivity studies of a series of \(\text{ N }_{2}\text{ O }_{2}\) chelated palladium(II) complexes Polyhedron 38 141
Negm N A, Badr E, Aiad I A, Zaki M F and Said M M 2012 Investigation the inhibitory action of novel diquaternary Schiff dibases on the acid dissolution of carbon steel in 1M hydrochloric acid solution Corros. Sci. 65 77
Sorkhabi H A, Shaabani B and Seifzadeh D 2016 Effect of some pyrimidinic Shciff bases on the corrosion of mild steel in hydrochloric acid solution Electrochim. Acta 50 3446
Yan Ji, Xu Bin, Gong W, Zhang X, Jin X, Ning W, Meng Y, Yang W and Chen Y 2016 Corrosion inhibition of a new Schiff base derivative with two pyridine rings on Q235 mild steel in 1.0M HCl J. Tai. Inst. Chem. E 66
Hegazy M A 2009 A novel Schiff base-based cationic gemini surfactants: Synthesis and effect on corrosion inhibition of carbon steel in hydrochloric acid solution Corros. Sci. 51 2610
Singh G S and Mmolotsi B J 2005 Synthesis of 2-azetidinones from 2-diazo-1, 2-diarylethanones and \(N\)-(2-thienylidene)imines as possible antimicrobial agents Il Farmaco 60 727
Navarro G, Perez de Vega M J, Garcia-Lopez M T, Andre I G, Snoeck R, Clercq E, Balzarini De and Muniz R G 2005 From 1-acyl-beta-lactam human cytomegalovirus protease inhibitors to 1-benzyloxycarbonylazetidines with improved antiviral activity. A straightforward approach to convert covalent to noncovalent inhibitors J. Med. Chem. 48 2612
Cheng Q, Kiyota H, Yamaguchi M, Horiguchi T and Oritani T 2003 Synthesis and biological evaluation of 4-deacetoxy-1,7-dideoxyazetidine paclitaxel analogue Bioorg. Med. Chem. Lett. 13 1075
Murad K, Deeb A and Kandil F 2014 Synthesis, Characterisation of some 2-azetidinonederivatives from 2,6-diaminopyridine and evaluation of their antimicrobial activity Int. J. Chem. Tech. Res. 7 3762
ASTM G 31–72 1990 (American Society for Testing and Materials: Philadelphia PA)
Lagrenee M, Mernari B, Bouanis M, Traisnel M and Bentiss F 2002 Study of the mechanism and inhibiting efficiency of 3,5-bis(4-methylthiophenyl)-4H-1,2,4-triazole on mild steel corrosion in acidic media Corros. Sci. 44 573
Becke A D 1993 Density-functional thermochemistry. III. The role of exact exchange J. Chem. Phys. 98 5648
Becke A D 1998 Density-functional exchange-energy approximation with correct asymptotic behavior Phys. Rev. A 38 3098
Palaniappan N, Chowhan L R, Jothi S, Bosco I G and Cole I S 2017 Corrosion inhibition on mild steel by phosphonium salts in 1 M \(\text{ HNO }_{3}\) aqueous medium Surf. Interfaces 6 237
Olasunkanmi L O, Obot I B, Kabanda M M and Ebenso E E 2015 Some Quinoxalin-6-yl Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid: Experimental and Theoretical Studies J. Phys. Chem. C 119 16004
Obot I B, Kaya S, Kaya C and Tüzün B 2016 Theoretical evaluation of triazine derivatives as steel corrosion inhibitors: DFT and Monte Carlo simulation approaches Res. Chem. Intermed. 42 4963
Martinez S 2003 Inhibitory mechanism of mimosa tannin using molecular modeling and substitutional adsorption isotherms Mater. Chem. Phys. 77 97
Obot I B and Gasem Z M 2014 Theoretical evaluation of corrosion inhibition performance of some pyrazine derivatives Corros. Sci. 83 359
Obot I B, Kaya S, Kaya C and Tüzün, B 2016 Density Functional Theory (DFT) modeling and Monte Carlo simulation assessment of inhibition performance of some carbohydrazide Schiff bases for steel corrosion Physica E 80 82
Kumar A M, Babu R S, Obot I B and Gasem Z M 2015 Fabrication of nitrogen doped graphene oxide coatings: experimental and theoretical approach for surface protection RSC Adv. 5 19264
Dohare P, Chauhan D S, Hammouti B and Quraishi M A 2017 Experimental and DFT Investigation on the Corrosion Inhibition Behavior of Expired Drug Lumerax on Mild Steel in Hydrochloric Acid Anal. Bioanal. Electrochem. 9 762
Wadhwani P M, Ladha D G, Panchal V K and Shah N K 2015 Enhanced Corrosion Inhibitive Effect of P-methoxybenzylidene-4,4-dimorpholine Assembled on Nickel Oxide Nanoparticles for Mild Steel in Acid Medium RSC Adv. 5 7098
Dohare P, Ansari K R, Quraishi M A and Obot I B 2017 Pyranpyrazole derivatives as novel corrosion inhibitors for mild steel useful for industrial pickling process: Experimental and Quantum Chemical study J. Ind. Eng. Chem. 52 197
Guan N M, Xueming L and L Fei 2004 Synergistic inhibition between o-phenanthroline and chloride ion on cold rolled steel corrosion in phosphoric acid Mater. Chem. Phys. 86 59
Sahin M, Bilgic S and Yilmaz H 2002 The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl medium Appl. Surf. Sci. 195 1
Larabi L, Harek Y, Benali O and Ghalem S 2005 Hydrazide derivatives as corrosion inhibitors for mild steel in 1M HCl Prog. Org. Coat. 54 256
Ghareba S and Omanovic S 2010 Interaction of 12-aminododecanoic acid with a carbon steel surface: Towards the development of ‘green’ corrosion inhibitors Corros. Sci. 52 2104
Solmaz R 2014 Investigation of corrosion inhibition mechanism and stability of Vitamin B1 on mild steel in 0.5M HCl solution Corros. Sci. 81 75
Solmaz R 2014 Investigation of adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by 5-(4-Dimethylaminobenzylidene)rhodanine Corros. Sci. 79 169
Hu K, Zhuang J, Zheng C, Ma Z, Yan L, Gu H, Zeng X and Ding J 2016 Effect of novel cytosine-l-alanine derivative based corrosion inhibitor on steel surface in acidic solution J. Mol. Liq. 222 109
John S, Kuruvilla M and Joseph A 2013 Adsorption and Inhibition Effect of Methyl Carbamate on Copper Metal in \(1 \text{ N } \text{ HNO }_{3}\): An Experimental and Theoretical Study RSC Adv. 3 8929
Daoud D T, Douadi T, Issaadi S and Chafaa S 2014 Adsorption and corrosion inhibition of new synthesized thiophene Schiff base on mild steel X52 in HCl and \(\text{ H }_{2}\text{ SO }_{4}\) solutions Corros. Sci. 79 50
Ehsani A, Mahjani M G, Moshrefi R, Mostaanzadeha H and Shayehb J S 2014 Electrochemical and DFT Study on the Inhibition of 316L Stainless Steel Corrosion in Acidic Medium by 1-(4-nitrophenyl)- 5-amino-1H-tetrazole RSC Adv. 4 20031
Ehteshamzadeh M, Jafari A H, Naderia E and Hosseini M G 2009 Effect of carbon steel microstructures and molecular structure of two new Schiff base compounds on inhibition performance in 1M HCl solution by EIS Mater. Chem. Phys. 113 986
Moradi M, Duan J and Du X 2013 Investigation of the effect of 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one inhibition on the corrosion of carbon steel in Bacillus sp. inoculated artificial seawater Corros. Sci. 69 338
Tang Y, Zhang F, Huc S, Cao Z, Wu Z and Jing W 2013 Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: Gravimetric, electrochemical, SEM and XPS studies Corros. Sci. 74 271
Pearson R G 1988 Absolute Electronegativity and Hardness: Application to Inorganic Chemistry Inorg. Chem. 27 734
Gomez B, Likhanova N V, Dominguez M A, Martinez-Palou R, Vela A and Gazquez J L 2006 Quantum Chemical Study of the Inhibitive Properties of 2-pyridyl-azoles J. Phys. Chem. B 110 8928
Naderi E, Jafari A H, Ehteshamzadeha M and Hosseini M G 2009 Effect of carbon steel microstructures and molecular structure of two new Schiff base compounds on inhibition performance in 1M HCl solution by EIS Mater. Chem. Phys. 115 852
Quraishi M A, Rafiquee M Z A, Khan S and Saxena N 2007 Corrosion inhibition of aluminium in acid solutions by some imidazoline derivatives J. Appl. Electrochem. 37 1153
Umoren S A, Obot I B, Israel A U, Asuquo P O, Solomon M M, Eduok U M and Udoh A P 2014 Inhibition of Mild Steel Corrosion in Acidic Medium using Coconut Coir Dust Extracted from Water and Methanol as Solvents J. Ind. Eng. Chem. 20 3612
Yadav D K, Maiti B and Quraishi M A 2010 Electrochemical and quantum chemical studies of 3,4-dihydropyrimidin-2(1H)-ones as corrosion inhibitors for mild steel in hydrochloric acid solution Corros. Sci. 52 3586
Dutta A, Panja S S, Nandi M M and Sukul D 2015 Effect of optimized structure and electronic properties of some benzimidazole derivatives on corrosion inhibition of mild steel in hydrochloric acid medium: Electrochemical and theoretical studies J. Chem. Sci. 127 921
Olasunkanmi L O, Kabanda M M and Ebenso E E 2016 Quinoxaline derivatives as corrosion inhibitors for mild steel in hydrochloric acid medium: Electrochemical and quantum chemical studies Phys. E 76 109
Lu T and Chen F 2012 Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm J. Mol. Graphics Modell. 38 314
Zhang Q B and Hua Y X 2009 Corrosion inhibition of mild steel by alkylimidazolium ionic liquids in hydrochloric acid Electrochim. Acta 54 1881
Zhao P, Liang Q and Li Y 2005 Electrochemical, SEM/EDS and quantum chemical study of phthalocyanines as corrosion inhibitors for mild steel in 1mol/l HCl Appl. Surf. Sci. 252 1596
Zeng J P, Zhang J Y and Gong X D 2011 Molecular dynamics simulation of interaction between benzotriazoles and cuprous oxide crystal Compt. Theo. Chem. 963 110
Kaya S, Tüzün B, Kaya C and Obot I B 2016 Determination of corrosion inhibition effects of amino acids: Quantum chemical and molecular dynamic simulation study J. Taiwan Inst. Chem. Eng. 58 528
Obot I B, Umoren S A, Gasem Z M, Suleiman R and El Ali B 2015 Theoretical prediction and electrochemical evaluation of vinylimidazole andallylimidazole as corrosion inhibitors for mild steel in 1M HCl J. Ind. Eng. Chem. 21 1328
Ramaganthan B, Gopiraman M, Olasunkanmi L O, Kabanda M M, Yesudass S, Bahadur I, Adekunle A S, Obot I B and Ebenso E E 2015 Synthesized photo cross linking chalcones as novel corrosion inhibitors for mild steel in acidic medium: experimental, quantum chemical and Monte Carlo simulation studies RSC Adv. 5 76675
Lukovits I, Kalman E. and Zucchi F 2001 Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency Corrosion 57 3. https://doi.org/10.5006/1.3290328
Acknowledgements
PD gratefully acknowledges the financial support of the Ministry of Human Resources and Development (MHRD), New Delhi for providing the Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dohare, P., Quraishi, M.A. & Obot, I.B. A combined electrochemical and theoretical study of pyridine-based Schiff bases as novel corrosion inhibitors for mild steel in hydrochloric acid medium. J Chem Sci 130, 8 (2018). https://doi.org/10.1007/s12039-017-1408-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-017-1408-x