Abstract
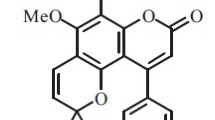
An efficient method for the preparation of 8-substituted odoratine [(3-(3 ′, 4 ′-methylenedioxyphenyl)-5,6,7-trimethoxyisoflavone] derivatives, structurally similar to glaziovianin A, a known cytotoxic substance, has been described. The key steps in the synthesis are site selective bromination reaction followed by Suzuki coupling reaction in very good yield. The structural assignment of the bromo derivative was determined utilizing 2D-HMBC and NOEs NMR techniques.

An efficient method for the preparation of odoratine, a naturally occurring isoflavone, has been described. 8-substituted odoratine derivatives were prepared via the Suzuki coupling reaction. Site selective bromination reaction was explored to obtain the key intermediates required for the coupling reactions.




Similar content being viewed by others
References
Kinoshita T, Ichinose K and Sankawa U 1990 Tetrahedron Lett. 31 7355
Singh O V and Muthukrishnan M 2005 Ind. J. Chem. 44B 2575
Yokosuka A, Haraguchi M, Usui T, Kazami S, Osada H, Yamori T and Mimaki Y 2007 Bioorg. Med. Chem. Lett. 17 3091
Hayakawa I, Shioda S, Ikedo A and Kogishi H 2014 Bull. Chem. Soc. Jpn. 87 544
Chinen T, Kazami S, Nagumo Y, Hayakawa I, Ikedo A, Takagi M, Yokosuka A, Imamoto N, Mimaki Y, Kigoshi H, Osada H and Usui T 2013 ACS Chem. Bio. 8 884
Ikedo A, Hayakawa I, Usui T, Kazami S, Osada H and Kigoshi H 2010 Bioorg. Med. Chem. Lett. 20 5402
Hayakawa I, Ikedo A and Kigoshi H 2007 Chem. Lett. 36 1382
Hayakawa I, Ikedo A, Chinen T, Usui T and Kigoshi H 2012 Bioorg. Med. Chem. 20 5745
Boland G M and Donnelly D M 1998 Nat. Prod. Rep. 241
Li Y H, Fu H G, Su F, Gao L M, Tang S, Bi C W, Li Y H, Wang Y X and Song D Q 2013 Chemistry Central J. 7 117
Mukne A P, Viswanathan V and Phadatare A G 2011 Pharmacogn. Rev. 5 13
Malla P, Kumar R and Kumar M 2013 Chem. Biol. Drug Des. 82 71
Shim Y S, Kim K C, Chi D Y, Lee K H and Cho H 2003 Bioorg. Med. Chem. 13 2561
Kim J, Seunghee H. and Sungwoo H. 2011 Bioorg. Med. Chem. Lett. 21 6977
Shim Y S, Kim K C, Lee K A, Shrestha S, Lee K H, Kim C K and Cho H 2005 Bioorg. Med. Chem. 13 1325
Griffiths L A 1962 J. Expt. Bot. 13 169
Haskins F A and Gorz H 1963 J. Science 139 496
Lehn J M and Ourisson G 1962 Bull. Soc. Chim. France 1133
Campbell R V M and Tannock J 1973 J. Chem. Soc. Perkin Trans. I 2222
Ngamga D, Yankep E, Tane P, Bezabih M, Ngadjui B T, Fomum Z T and Abegaz B M 2005 Z. Naturforsch., B: Chem. Sci. 60b 973
Combes S, Finet J P and Siri D 2002 J. Chem. Soc. Perkin Trans. I 38
Yankep E, Njamen D, Fotsing M T, Fomum Z T, Mbanya J C, Giner R M, Recio M C, Manez S and Rios J L 2003 J. Nat. Prod. 66 1288
Antus S, Farkas L, Kardos-Balogh Z and Nogradi M 1975 Chem. Ber. 108 3883
Nakano T, Alonso J, Grillet R and Martin A 1979 J. Chem. Soc. Perkin Trans. I 2107
Krishnamurti M and Seshagiri S N 1976 Ind. J. Chem. 14B 222
Chatterjea J N, Shaw S C and Fatma A 1985 J. Ind. Chem. Soc. 62 990
Bhardwaj D K, Jain R K, Malhotra V P and Sharma A K 1983 Proc. Ind. Nat. Sci. Acad. 49A 168
(a) Ravi Kumar P, Behera M, Sambaiah M, Venu K, Nagaraju P, Jaya Shree A and Satyanarayana Y 2014 J. Amino Acid ID 721291; (b) Ravi Kumar P, Behera M, Raghavulu K, Jaya Shree A and Satyanarayana Y 2012 Tetrahedron Lett. 53 4108
Elassar Abdel-Zaher A and El-Khair Adel A 2003 Tetrahedron 59 8463
Biegasiewicz K F, Denis J D, Carrol V M and Priefer R 2010 Tetrahedron Lett. 51 4408
Miyaura N and Suzuki A 1995 Chem. Rev. 95 2457
Kotha S, Lahiri K and Dhurke K 2002 Tetrahedron 58 9633
Acknowledgements
Authors are grateful to GVK Biosciences management for the financial support. We thank Dr. Sudhir Kumar Singh for encouragement and motivation. Help from the analytical department for the analytical data is appreciated. PKH gratefully acknowledges UGC, New Delhi for providing UGC-FRPS research start-up grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information (SI)
The spectroscopic data (1H-NMR, 13C-NMR, IR and HRMS) of synthesized compounds are presented in the Supplementary Information, available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
KUMAR, P.R., BALAKRISHNA, C., MURALI, B. et al. An efficient synthesis of 8-substituted Odoratine derivatives by the Suzuki coupling reaction. J Chem Sci 128, 441–450 (2016). https://doi.org/10.1007/s12039-016-1042-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1042-z