Abstract
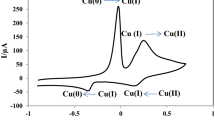
A non-enzymatic hydrogen peroxide sensor was developed using gold@platinum nanoparticles (Au@PtNPs) with core@shell structure fabricated on glassy carbon electrode (GCE) by electroless deposition method. Initially, gold nanoparticles (AuNPs) were deposited on GCE by reducing HAuCl4 in the presence of NH2OH and the deposited AuNPs on GCE act as the nucleation centre for the deposition of platinum nanoparticles (PtNPs) in the presence of H2PtCl6 and NH2OH. SEM and AFM studies demonstrated that the electroless deposition of Pt on Au was isotropic and uniform. Further, Au@PtNP-modified substrates were characterized by X-ray photoelectron spectroscopy (XPS), energy dispersive X-ray analysis (EDAX) and cyclic voltammetry (CV). XPS showed characteristic binding energies at 71.2 and 74.4 eV for PtNPs and, 83.6 and 87.3 eV for AuNPs indicating the zero-valent nature in both of them. The electrocatalytic activity of Au@PtNP-modified electrode was investigated towards hydrogen peroxide (HP) reduction. The modified electrode exhibited higher electrocatalytic activity towards HP by not only shifting its reduction potential by 370 mV towards less positive potential but also by enhancing the reduction current when compared to bare and AuNP-modified GCE. The present method shows better sensitivity compared to the reported methods in literature and the detection limit was found to be 60 nM.

A non-enzymatic hydrogen peroxide sensor was developed using gold@platinum nanoparticles with core@shell structure fabricated on glassy carbon electrode by electroless deposition method.









Similar content being viewed by others
References
Wang Y H, Yang X J, Bai J, Jiang X and Fan G Y 2013 Biosens. Bioelectron. 43 180
Sato K, Abe E, Takahashi M and Anzai J 2014 J. Colloid Interf. Sci. 432 92
Liu W N, Ding D, Song Z L, Bian X, Nie X K, Zhang X B, Chen Z and Tan W H 2014 Biosens. Bioelectron. 52 438
Ahmad M, Pan C, Gan L, Nawaz Z and Zhu J 2010 J. Phys. Chem. C 114 243
Usui Y, Sato K and Tanaka M 2003 Angew. Chem. Int. Ed. 42 5623
Halliwell B, Clement M V and Long L H 2000 FEBS Lett. 486 10
Long L H, Evans P J and Halliwell B 1999 Biochem. Biophys. Res. Commun. 262 605
Dezwart L L, Meerman J H N, Commandeur J N W and Vermeulen W P E 1999 Free Radic. Biol. Med. 26 202
Ensafi A A, Atashbar N Z, Ghiaci M, Taghizadeh M and Rezaei B 2015 Mat. Sci. Eng. C 47 290
Santucci R, Laurenti E, Sinibaldi F and Ferrari R P 2002 Biochim. Biophys. Acta 1596 225
Klassen N V, Marchington D and Mcgowan H C E 1994 Anal. Chem. 66 2921
Mori I, Takasaki K, Fujita Y and Matsuo T 1998 Talanta 47 631
Greenway G M, Leelasattarathkul T, Liawruangrath S, Wheatley R A and Youngvises N 2006 Analyst 131 501
Pinkernell U, Effkemann S and Karst U 1997 Anal. Chem. 69 3623
Luque G L, Ferreyra N F, Leyva A G and Rivas G A 2009 Sens. Actuat. B 142 331
Guascito M R, Filippo E, Malitesta C, Manno D, Serra A and Turco A 2008 Biosens. Bioelectron. 24 1057
Guascito M R, Chirizzi D, Malitesta C, Mazzotta E, Siciliano M, Siciliano T, Tepore A and Turco A 2011 Biosens. Bioelectron. 26 3562
Lei C X, Hu S Q, Shen G L and Yu R Q 2003 Talanta 59 981
Xu Q, Zhao Y, Xu J Z and Zhu J J 2006 Sens. Actuat. B 114 379
Bai Y, Wang Y D, Zheng W J and Chen Y S 2008 Colloids Surf. B 63 110
Pumera M, Merkoci A and Alegret S 2006 Sens. Actuat. B 113 617
Song M J, Hwang S W and Whang D M 2010 Talanta 80 1648
Palanisamy S, Chen S M and Sarawathi R 2012 Sens. Actuat. B 166–167 372
Wang L, Bo X, Bai J, Zhu L and Guo L 2010 Electroanalysis 22 2536
Campbell C T 1990 Annu. Rev. Phys. Chem. 41 775
Rodriguez J A 1996 Surf. Sci. Rep. 24 225
Luo J, Maye M M, Kariuki N N, Wang L Y, Njoki P, Lin Y, Schadt M, Naslund H R and Zhong C J 2005 Catal. Today 99 91
Kang X H, Mai Z B, Zou X Y, Cai P X and Mo J Y 2007 Anal. Biochem. 369 71
Kristian N, Yan Y and Wan X 2008 Chem. Commun. 353
Bond G C 2007 Platinum Met. Rev. 51 63
Harada M, Asakura K and Toshima N 1993 J. Phys. Chem. 97 5103
Heng H Y, Gibbons P C, Kelton K F and Buhro W E 1997 J. Am. Chem. Soc. 119 10382
Baletto F, Mottet C and Ferrando R 2003 Phys. Rev. Lett. 90 135504
Crooks R and Zhao M 1999 Adv. Mat. 11 217
Jena B K and Raj C R 2007 Langmuir 23 4064
Jena B K and Raj C R 2008 Chem. Mater. 20 3546
Ye H and Crooks R M 2005 J. Am. Chem. Soc. 127 4930
Kumar S and Zou S 2005 J. Phys. Chem. B 109 15707
Lou Y, Maye M M, Han L, Luo J and Zhong C. 2001 Chem. Commun. 473
Zhou Y, Yu G, Chang F, Hu B and Zhong C J 2012 Anal. Chim. Acta 757 56
Wang J, Gao H, Sun F and Xu C 2014 Sens. Actuat. B 191 612
Gowthaman N S K and John S A 2015 RSC Adv. 5 42369
Cao L, Tong L, Diao P, Zhu T and Liu Z 2004 Chem. Mater. 16 3239
Liz-Marzan L M and Philipse A P 1995 J. Phys. Chem. 99 15120
Matsumoto T, Komatsu T, Arai K, Yamazaki T, Kijima M, Shimizu H, Takasawab Y and Nakamura J 2004 Chem. Commun. 840
Kannan P and John S A 2010 Electrochim. Acta 55 3497
Li Y, Lu Q, Wua S, Wanga L and Shi X 2013 Biosens. Bioelectron. 41 576
Li Y, Ma J and Ma Z 2013 Electrochim. Acta 108 435
Kang Q, Yang L X and Cai Q Y 2008 Bioelectrochemistry 74 62
Xu C, Sun F, Gao H and Wang J 2013 Anal. Chim. Acta 780 20
Heli H, Sattarahmady N, Vais R D and Mehdizadeh A R 2014 Sens. Actuat. B 192 310
Acknowledgements
NSKG thanks the University Grants Commission (UGC), New Delhi, for the award of a Meritorious Student Fellowship (F. 7-225/2009(BSR) dt. 15.01.2013). Financial support from Department of Biotechnology (BT/PR10372/PFN/20/904/2013), New Delhi, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor R. Ramaraj on the occasion of his 60th anniversary
Supplementary Information (SI)
UV-Vis absorption spectra and EDAX spectrum obtained for ITO/Au@PtNPs, CVs obtained for GCE/AuNPs at different deposition times, HP reduction at GCE/Au@PtNPs at different deposition times, effect of scan rate and concentration on HP reduction at GC/Au@PtNP electrode and amperometric determination of HP oxidation are given in the supporting information. Supplementary Information is available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
GOWTHAMAN, N.S.K., JOHN, S.A. Electroless deposition of Gold-Platinum Core@Shell Nanoparticles on Glassy Carbon Electrode for Non-Enzymatic Hydrogen Peroxide sensing# . J Chem Sci 128, 331–338 (2016). https://doi.org/10.1007/s12039-016-1038-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1038-8