Abstract
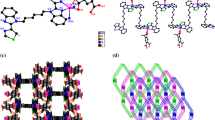
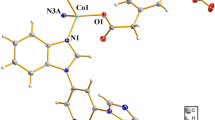
In this paper, we report two coordination polymers, [Cd(Medpq)(SO4) (H2O)2]n (1) and {[Mn(Medpq)(QUI)H2O] ⋅ H2O} n (2) (H2QUI = 2,3-pyridinedicarboxylic acid, Medpq = 2-methyldipyrido[3,2-f:2,3-h]quinoxaline) by hydrothermal processing and structural characterization by elemental analysis, thermogravimetric analysis and single-crystal X-ray diffraction. The coordination polymers 1 and 2 have 1D chains formed via coordination bonds, and unique two-dimensional supramolecular structures are further formed due to π- π stacking interactions. The results reveal that the coordination preferences of metal ions play a critical role in the framework construction of the two coordination polymers. Meanwhile, natural bond orbital (NBO) analysis was performed by the PBE0/LANL2DZ method in Gaussian 09 program. The calculated results show the obvious covalent interaction between the coordinated atoms and metal ions.

Two coordination polymers were prepared by the combination of two metal salts (Cd and Mn) and 2-methyldipyrido[3,2-f:2′,3′-h]quinoxaline ligands. The coordination polymers have 1D chains formed via coordination bonds, and unique two-dimensional supramolecular structures formed due to π-π stacking interactions. The results reveal that the coordination preference of the metal ion plays a critical role in the construction of the coordination polymer.








Similar content being viewed by others
References
Zhang X J, Wang L Y, Wei B, Zhang W T and Che G B 2015 Chinese J. Struct. Chem. 34 1069
Kaustuv B and Kumar B 2012 Cryst. Growth Des. 12 4264
Cook T R, Zheng Y R and Stang P J 2013 Chem. Rev. 113 734
McKinlay A C, Morris R E, Horcajada P, Férey G, Gref R, Couvreur P and Serre C 2010 Chem. Int. Ed. 49 6260
Jiang H L and Xu Q 2011 Chem. Commun. 47 3351
Furukawa H, Ko N, Go Y B, Aratani N, Choi S B and Yagh O M 2010 Science 329 424
Keefe M O and Yaghi O M 2012 Chem. Rev. 112 675
Vishnoi P, Kalita A C and Murugavel R 2014 J. Chem. Sci. 126 1385
Mudsainiyan R K, Jassal A, Arora M and Chawla S K 2015 J. Chem. Sci. 127 849
Suresh P and Prabusankar G 2014 J. Chem. Sci. 126 1409
Tripathi S, Srirambalaji R, Singh N and Anantharaman G 2014 J. Chem. Sci. 126 1423
Chen X L, Han Z X, Hu H M, Wang J J, Chen S H, Fu F, Li N, Yang M L and Xue G L 2009 Inorg. Chim. Acta 362 3963
Gillard R D, Lancashire R J and Williams P A 1979 Transition Met. Chem. 4 115
Wang X L, Chen Y Q, Liu G C, Zhang J X, Lin H Y and Chen B K 2009 Inorg. Chim. Acta 362 3963
Huang Y J, Yan Y S, Pan Y R and Zhang H Y 2015 J. Clust. Sci. 26 925
Sheldrick G M, SHELXS 97, 1997 Program for the Solution of Crystal Structure (Göttingen: University of Göttingen)
Sheldrick G M, SHELXL 97, 1997 Program for the Refinement of Crystal Structure (Göttingen: University of Göttingen)
Frisch M J, et al. 2009 Gaussian 09, revision B.09 (Pittsburgh, PA: Gaussian, Inc.)
Parr R G and Yang W 1989 In Density functional theory of atoms and molecules (Oxford: Oxford University Press)
(a) Ernzerhof M and Scuseria G E 1999 J. Chem. Phys. 110 5029; (b) Adamo C and Barone V 1999 J. Chem. Phys. 110 6158
Dunning T H Jr and Hay P J 1976 In Modern Theoretical Chemistry Schaefer H F III (Ed.) (New York: Plenum) pp 1–28
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
CCDC 1442203 and1442204 contain the supplementary crystallographic data for the molecules reported in this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif, fax: + 44 1223 336 033 or by e-mail: deposit@ccdc.cam.ac.uk. Selected bond lengths and angles, molecular orbitals figures, CIF and checkCIF files are available as electronic Supplementary Information for this paper at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
HUANG, YJ., PAN, YR., DU, G. et al. Extended structures of two coordination polymers based on 1,10-phenanthroline derivatives: Preparation, structural characterization and properties. J Chem Sci 128, 459–465 (2016). https://doi.org/10.1007/s12039-016-1035-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1035-y