Abstract
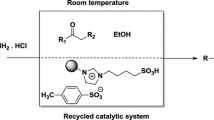
A new approach for the synthesis of benzimidazoles using ionogel under solvent-free conditions is reported. Catalytic activity of ionogel was compared with silica coated with ionic liquid (silica-IL) and it was found that ionogel is highly active compared to silica-IL for the synthesis of benzimidazoles. Moreover, ionogel was recyclable for the synthesis of benzimidazoles.

An efficient synthetic approach for the synthesis of benzimidazoles catalyzed by highly active ionogel under solvent-free conditions has been described.





Similar content being viewed by others
References
Desikan S and Doraiswamy L K 1995 Ind. Eng. Chem. Res. 34 3524; (b) McMorn P and Hutchings G J 2004 Chem. Soc. Rev. 33 108
Spasov A A and Yozhitsa I N 1999 J. Pharm. Chem. 33 232
Mavrova A T S, Anichina K K and Vuchev D I 2005 Bioorg. Med. Chem. 13 5550
Goker H, Kus C and Boykin D W 2002 Bioorg. Med. Chem. 10 2589
Goker H, Kus C and Boykin D W 2005 Eur. J. Med. Chem. 40 1062
Andrzejewska M and Yepez M L 2002 Eur. J. Med. Chem. 37 973
Ozden S, Atabey D and Goker H 2005 Bioorg. Med. Chem. 13 1587
Ramla M M and Omar M A 2006 Bioorg. Med. Chem. 14 7372
Boiani M and Gonzalez M 2005 Mini Rev. Med. Chem. 5 409
Niknam K and Raviz A F 2007 J. Iran Chem. Soc. 4 438
Bhatt A K, Karadia H G, Shah P R, Parmar M P and Patel H D 2004 Ind. J. Heterocycl. Chem. 13 281
Kim Y, Kumar M R, Park N, Heo Y and Lee S 2011 J. Org. Chem. 76 9577
Jacob R G, Dutra L G, Radatz C S, Mendes S R, Perin G and Lenardao E J 2009 Tetrahedron Lett. 50 1495
Oda S, Shimizu H, Aoyama Y, Ueki T, Shimizu S, Osato H and Takeuchi Y 2012 Org. Process Res. Dev. 16 96
Prabhakar V, Babu K S, Ravindranath L K and Latha J 2015 World J. Pharmacy Pharma. Sci. 4 553
Sharma P, Gupta M, Kant R and Gupta V K 2015 New J. Chem. 39 5116
Sharma P and Gupta M 2015 Green Chem. 17 1100
Valdez J, Cedillo R, Campos A H, Yepez L, Luis F H, Vazquez G N, Tapia A, Cortes R, Hernandez M and Castillo R 2002 Bioorg. Med. Chem. Lett. 12 2221
Hein D W, Alhein R J and Leavitt J J 1957 J. Am. Chem. Soc. 79 427
Devalla V R and Ethirajulu K 1995 J. Chem. Soc. Perkin Trans. 2 1497
Eshghi H, Rahimizadeh M, Sedaghat P and Shiri A 2012 Bull. Korean Chem. Soc. 33 515
Gadekar S L, Arbad R B and Lande K M 2010 Chin. Chem. Lett. 219 1053
Latif N, Mishriky N, Assad F M and Meguid S B 1982 Indian J. Chem. 21B 872
Bougrin K, Loupy A and Soufiaoui M 1998 Tetrahedron 54 8055
Mahdavinia H G, Amani A M, Rostamizadeh S and Sepehrian H 2012 Heterocycl. Commun. 18 33
Navarrete V G, Moreno D H, Estrada S S, Torres P M, Leon R I, Tlahuext H, Munoz M O and Torres G H 2007 Synth. Commun. 37 2815
Ramsden Christopher A and Rose Helen L 1997 J. Chem. Soc. Perkin Trans. 1 2319
Acknowledgements
We are sincerely thankful to the Director, IIIM Jammu and SAIF Chandigarh for facilities for spectra; Head, Central Research facility section, IIT Roorkee for SEM and TEM. We also thank the Department of Chemistry, University of Jammu, for NMR, FTIR and TGA analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Supplementary file contains details on preparation of the ionic liquid and its characterization, preparation of the ionogel and silica-supported catalyst, characterization of the ionogel including SEM, TEM, FTIR and TGA. It also contains spectral data of the synthesized compounds.
Supplementary Information is available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SHARMA, P., GUPTA, M. 1,3,5-Trimethylpyrazolium chloride based ionogel as an efficient and reusable heterogeneous catalyst for the synthesis of benzimidazoles. J Chem Sci 128, 61–65 (2016). https://doi.org/10.1007/s12039-015-1000-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-1000-1