Abstract
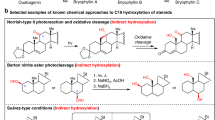
Several microbial transformations of steroids to 17-keto cortisones through side chain cleavage have been presented in the literature; however, yields and product selectivity in these methods were low. In the present study, some new methods have been identified for the side chain cleavage of prednisolone (1) to form 11- β-hydroxy-1,4-androstadiene-3,17-dione (11- β-hydroxy ADD). Prednisolone upon reaction with zinc chloride in dry THF results in the formation of cleavage product in good yield (76%). 11- β-hydroxy ADD (2) has been formed in moderate yield (60%) under the Reformatsky reaction conditions by reacting with zincate. While performing the Wittig reaction using stable ylides, again results in the formation of compound 2 in good yield (56%). Side chain cleavage of prednisolone was confirmed from the physical and analytical data and similar when compared with the literature reports.

A few chemical methods were investigated for the side chain cleavage of prednisolone in order to obtain 11-β-hydroxy-1,4-androstadiene-3,17-dione. Among these methods, ZnCl2 mediated transformation gives the best results with 76% yield.




Similar content being viewed by others
References
(a) Reese P B 2001 Steroids 66 481; (b) Charney W and Herzog H L 1967 In Microbial Transformations of Steroids (New York: Academic Press) p. 5; (c) Mahato S B and Banerjee S 1984 Phytochemistry 23 2131; (d) Mahato S B and Banerjee S 1985 Phytochemistry 24 1403; (e) Mahato S B, Banerjee S and Podder S 1989 Phytochemistry 28 7
(a) Malaviya A and Gomes J 2008 Bioresour. Technol. 996725; (b) Sallam L A R, El-Refai A M and El-Minofi H A 2003 Process Biochem. 40 203
(a) Manosroi A, Saowakhon S and Manosroi J 2008 J. Steroid Biochem. Mol. Biol. 108 132; (b) Sedlaczek L 1988 Crit. Rev. Biotechnol. 7 187; (c) Fernandes P, Cruz A, Angelova B, Pinheiro H M and Cabral J M S 2003 Enzyme Microb. Technol. 32 688; (d) Mahato S B and Garai S 1997 Steroids 62 332
(a) Eshrat G F and Aroona C 2011 Res. J. Microbiol. 6 98; (b) Eshrat G F, Tabatabaei-Yazdi M, Faramarzi M A and Amini M 2006 J. Sci. Islamic Repub. Iran 17 309; (c) Bartmanska A and Gładysz J D 2007 Enzyme Microb. Technol. 40 1615; (d) Choudhary M I, Musharraf S G and Shaheen F 2002 Nat. Prod. Lett. 16 377; (e) Andrushina V A, Druzhinina A V, Yaderets V V, Stitsenko T S and Voishvillo N E 2011 Appl. Biochem. Microb. 47 42
Malaviya A and Gomes J 2008 Bioresour. Technol. 99 6725
Ahmed F,Williams R A D and K E Smith 1996 J. Steroid Biochem. Molec. Biol. 58 337
Bartmanska A and Gładysz D J 2007 Enzyme Microb. Technol. 40 1615
Wadhwa L and Smith K E 2000 FEMS Microbiol. Lett. 192 179
(a) Akhrem A A and Titov Y A 1970 In Steroids and Microorganisms (Moscow: Nauka) p. 526; (b) Fernandes P, Cruz A, Angelova B, Pinheiro H M and Cabral J M S 2003 Enzyme Microb. Technol. 32 688
Perez C, Falero A, Hung B R, Tirado S and Balcinde Y 2005 J. Ind. Microbiol. Biotechnol. 32 83
Lin Y, Song X, Fu J, Lin J and Qu Y 2009 Bioresour. Technol. 100 1864
Fernandes P, Cruz A, Angelova B, Pinheiro H M and Cabral J M S 2003 Enzyme Microb. Technol. 32 688
Donova M V, Egorova O V and Nikolayeva V M 2005 Process Biochem. 40 2253
Simons Jr. S S, Merchlinsky M J and Johnson D F 1981 Steroids 37 281
Pera A L, Leggio A, Siciliano C, Gioia M L D, Napoli A, Sindona G and Liguori A 2003 Steroids 68 139
Pinto R M A, Salvador J A R, Roux C L and Paixao J A 2009 J. Org. Chem. 74 8488
Acknowledgements
The authors thank Dr. G. N. Qazi, Hon’ble Vice Chancellor, Jamia Hamdard- New Delhi for providing the facilities. SS thanks Hamdard National Foundation for providing Hamdard Centenary Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Supplementary information is available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SURYA PRATAP, P.R., SHAFI, S., NAAZ, F. et al. Chemical methods for the conversion of Prednisolone to 11-β-hydroxy-1,4-androstadiene-3,17-dione. J Chem Sci 127, 1827–1830 (2015). https://doi.org/10.1007/s12039-015-0950-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0950-7