Abstract
The synthesis and characterization of low-valent organoselenium and tellurium derivatives of hexabromododecahydrotriphenylene has been attempted. The reaction of hexabromododecahydrotriphenylene with in situ generated disodium dichalcogenides (Na2E2; E = Se, Te) afforded insoluble chalcododecahydrotriphenylene derivatives, 20, 21 respectively. Attempted aromatization of 20 and 21 using DDQ (2, 3-dichloro-5, 6-dicyano-1,4-benzoquinone) as an oxidizing agent afforded the co-crystals. The hexaselenophenyl derivative, 22 and the hexaselenocyanate derivative 23 were synthesized by the reaction of in situ generated sodium arylselenolate/potassium selenocyanate with hexabromododecahydrotriphenylene, respectively. The compounds were characterized by common spectroscopic tools and a few by single crystal x-ray crystallographic studies.

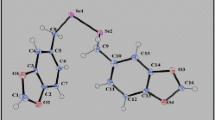
Organoselenide 20 was synthesized by the reaction of 1, 4, 5, 8, 9, 12-hexabromododecahydrotriphenylene with in situ generated Na2Se2 at room temperature. Aromatization of 20 afforded compounds 24 and 26.









Similar content being viewed by others
References
Wu Y-T and Siegel J S 2006 Chem. Rev. 106 4843
Tsefrikas V M and Scott L T 2006 Chem. Rev. 106 4868
Van Dijk J T M, Hartwijk A, Bleeker A C, Lugtenburg J and Cornelisse J 1996 J. Org. Chem. 61 1136
Sakurai H, Daiko T, Sakane H, Amaya T and Hirao T J. Am. Chem. Soc. 127 11580
Sakurai H, Daiko T and Hirao T 2003 Science 301 1878
Higashibayashi S and Sakurai H 2008 J. Am. Chem. Soc. 130 8592
Tanaka H N T and Mikawa H 1982 Chem. Lett 1841
Murschall R G H and Monch W 1982 Chem. Lett 727
Murschall R, Gant H and Mönch W 1982 Solid State Commun. 42 787
Li X, Zhu Y, Shao J, Wang B, Zhang S, Shao Y, Jin X, Yao X, Fang R and Shao X 2014 Angew. Chem. Int. Ed. 53 535
Wei J, Jia X, Yu J, Shi X, Zhang C and Chen Z 2009 Chem. Commun. 4714
Sheldrick G M SHELXS-97, Program for Crystal Structure Solution University of Göttingen, 1997
Sheldrick G M SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen, 1997
International Tables for X-ray Crystallography Vol 4 1974 (Birmingham: Kynoch Press)
Wei J, Gao Y, Ma X, Jia X, Shi X and Chen Z 2010 Chem. Commun. 46 3738
Selvakumar K, Singh H B, Goel N, Singh U P and Butcher R J 2011 Dalton Trans. 40 9858
Panda A, Menon S C, Singh H B and Butcher R J 2001 J. Organomet. Chem. 62 87
Müller J and Terfort A 2006 Inorg. Chim. Acta 359 4821
Klemm L H, Hall E, Cousins L and Klopfenstein C E 1987 J. Heterocyclic Chem 24 1749
Imamura K, Takimiya K, Otsubo T and Aso Y 1999 Chem. Commun. 1859
Srivastava K, Shah P, Singh H B and Butcher R J 2011 Organometallics 30 534
Ahmed F R and Trotter J 1963 Acta Cryst 16 503
Zyss J, Ledoux-Rak I, Weiss H -C, Blasser D, Boesse R, Thallapally P K, Thalladi V R and Desiraju G R 2003 Chem. Matt. 15 3063
Collings J C, Roscoe K P, Thomas R L, Batsanov A S, Stimson L M, Howard J A K and Marder T B 2001 New J. Chem. 25 1410
Patra A, Wijsboom Y H, Leitus G and Bendikov M 2009 Organic Letters 11 1487
Acknowledgements
HBS is grateful to the Department of Science and Technology (DST), New Delhi (India), for generous funding. PRP is thankful to Department of Chemistry, IIT Bombay for a teaching assistantship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
CCDC-1004393(3), CCDC-990989(3–24a), CCDC-99 0990(3–24b), CCDC-990991(3–24c), CCDC-990992 (3–26) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
PRASAD, P.R., SINGH, H.B. & BUTCHER, R.J. Isolation and structures of some selenium and tellurium derivatives of 1, 4, 5, 8, 9, 12-hexabromododecahydrotriphenylene as co-crystals of triphenylene. J Chem Sci 126, 1311–1321 (2014). https://doi.org/10.1007/s12039-014-0713-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0713-x