Abstract
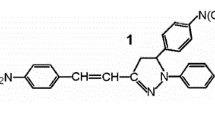
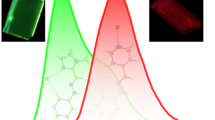
Salt formation has been shown as a simple strategy to induce aggregated induced emission or aggregated enhanced emission in primary ammonium salts derived from 9-anthracene carboxylic acid, 1-pyrene carboxylic acid, 3-coumarin carboxylic acid and histamine. All the salts displayed enhanced fluorescence in their solid state compared to that in their solution state. Single crystal structure of the salt of 9-anthracene carboxylic acid i.e., His-anthracene revealed that restricted intramolecular rotation of the fluorphoric moiety (anthracene) was responsible for such radiative pathway leading to enhanced emission.

A simple strategy of salt formation has been exploited to induce aggregation-induced emission or aggregation-enhanced emission in primary ammonium salts derived from 9-anthracene carboxylic acid, 1-pyrenecarboxylic acid, coumarin-3-carboxylic acid and histamine.





Similar content being viewed by others
References
Lakowicz J R 1999 In Principles of Fluorescence Spectroscopy (New York: Kluwer Academic/Plenum Publishers) p 6
Thomas III S W, Joly G D and Swager T M 2007 Chem. Rev. 107 1339
Barks B 1970 In Photophysics of Aromatic Molecules (London: Wiley)
Thompson R B 2006 In Fluorescence Sensors and Biosensors (Boca Raton: CRC)
Tang C W and Vanslyke S A 1987 Appl. Phys. Lett. 51 913
Geddes C D and Lakopwicz J R 2005 In Advanced Concepts in Fluorescence Sensing (Norwell: Springer)
Jares-Erijman E A and Jovin T M 2003 Nat. Biotechnol. 21 1387
Saigusa H and Lim E C 1995 J. Phys. Chem. 99 15738
Luo J, Xie Z, Lam J W Y, Cheng L, Chen H, Qiu C, Kwok H S, Zhan X, Liu Y, Zhu D and Tang B Z 2001 Chem. Commun. 1740
Tang B Z, Zhan X, Yu G, Lee P P S, Liu Y and Zhu D 2001 J. Mater. Chem. 11 2974
Wu Y T, Kuo M Y, Chang Y T, Shin C C, Wu T C, Tai C C, Cheng T H and Liu W S 2008 Angew. Chem. Int. Ed. 47 9891
Chen J, Law C C W, Lam J W Y, Dong Y, Lo S M F, Williams I D, Zhu D and Tang B Z 2003 Chem. Mater. 15 1535
Grimme S 2008 Angew. Chem. Int. Ed. 47 3430
Shimizu M, Takeda Y, Higashi M and Hiyama T 2009 Angew. Chem. Int. Ed. 48 3653
An B K, Kwon S K, Jung S D and Park S Y 2002 J. Am. Chem. Soc. 124 14410
Liu J, Lam J W Y and Tang B Z 2009 Chem. Rev. 109 5799
Qin A, Jim C K W, Tang Y, Lam J W Y, Liu J, Mahtab F, Gao P and Tang B Z 2008 J. Phys. Chem. B 112 9281
Dastidar P 2008 Chem. Soc. Rev. 37 2699
Acknowledgement
UKD thanks CSIR and IACS for fellowships. PD thanks IACS for infrastructure and financial support. Professor Amitava Patra, IACS is also thanked for solid state PL measurements in his laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
CCDC No. 1001247 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
DAS, U.K., DASTIDAR, P. Aggregation enhanced emission (AEE) in organic salt: A structure-property correlation based on single crystal studies. J Chem Sci 126, 1357–1362 (2014). https://doi.org/10.1007/s12039-014-0710-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0710-0