Abstract
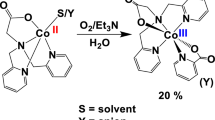
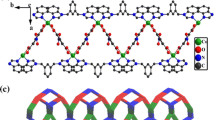
This work presents the syntheses, characterization and hydrogen bonding based self-assembly of Co3+ complexes of pyridine-amide based bidentate ligands containing appended phenol and catechol groups. Placement of multiple hydrogen bond donors (phenolic OH and amidic NH groups) and acceptors (Oamide groups) in these molecules results in interesting self-assembled architectures.

Cobalt complexes of pyridine-amide based bidentate ligands containing appended phenol and catechol groups are discussed. Placement of multiple hydrogen bond donors and acceptors in these coordination complexes results in interesting self-assembly.








Similar content being viewed by others
References
Kumar G and Gupta R 2013 Chem. Soc. Rev. 42 9403
(a) Desiraju G R 1995 Angew. Chem. Int. Ed. 34 2311; (b) Desiraju G R 2001 Nature 412 397; (c) Desiraju G R 2007 Cryst. Eng. Comm. 9 91; (d) Prins L J, Reinhoudt D N and Timmerman P 2001 Angew. Chem. Int. Ed. 40 2382; (e) Steiner T 2002 Angew. Chem. Int. Ed. 41 48; (e) Wuest J D 2005 Chem. Commun. 5830; (f) Suslick K S, Bhyrappa P, Chou J S, Kosal M E, Nakagaki S, Smithenry D W and Wilson S R 2005 Acc. Chem. Res. 38 283; (f) Beatty A M 2003 Coord. Chem. Rev. 246 131
Ali A, Hundal G and Gupta R 2012 Cryst. Growth Des. 12 1308
Kumar G, Aggarwal H and Gupta R 2013 Cryst. Growth Des. 13 74
(a) Sarma R J and Baruah J B 2007 Cryst. Growth Des. 7 989 and references cited therein; (b) Sarma R J and Baruah J B 2005 Cryst. Eng. Comm. 7 706; (c) Sarma R J and Baruah J B 2006 Chem. Eur. J. 12 4994
(a) Aitipanula S, Thallapally P K, Thaimattam R, Jaskolsiki M and Desiraju G R 2002 Org. Lett. 4 921; (b) Thallapally P K, Katz A K, Carrell H L and Desiraju G R 2002 Chem. Commun. 344; (c) Aitipanula S, Desiraju G R, Jaskolsiki M, Nangia A and Thaimattam R 2003 Cryst. Eng. Comm. 5 447; (d) Vangala V R, Mondal R, Broder C K, Howard J A K and Desiraju G R 2005 Cryst. Growth Des. 5 99
(a) Malek N, Maris T, Perron M E and Wuest J D 2005 Angew. Chem. Int. Ed. 44 4021; (b) Malek N, Maris T, Simard M and Wuest J D 2005 J. Am. Chem. Soc. 127 5910; (c) Saied O, Maris T, Wang X, Simard M and Wuest J D 2005 J. Am. Chem. Soc. 127 10008; (d) Fournier J H, Maris T, Simard M and Wuest J D 2003 Cryst. Growth Des. 3 535
(a) Tanaka K, Okada T and Toda F 1993 Angew. Chem. Int. Ed. 32 1147; (b) Urbanczyk-Lipkowska Z, Yoshizawa K, Toyota S and Toda F 2003 Cryst. Eng. Comm. 5 114; (c) Yoshizawa K, Toyota S, Toda F, Kato M and Csöregh I 2007 Cryst. Eng. Comm. 9 786; (d) Goldberg I, Stein Z, Kai A and Toda F 1987 Chem. Lett. 1617; (e) Toda F, Tanaka K, Hyoda T and Mak T C W 1988 Chem. Lett. 107
(a) Thakuria R, Sarma B and Nangia A 2010 New J. Chem. 34 623; (b) Aitipamula S and Nangia A 2005 Chem.–Eur. J. 11 6727; (c) Sarma B, Roy S and Nangia A 2006 Chem. Commun. 4918; (d) Aitipanula S and Nangia A 2005 Chem. Commun.3159;(e) Jagadeesh, N and Nangia A 2006 Cryst. Growth Des. 6 1995
(a) Kobayashi K, Shirasaka T, Horn E and Furukawa N 2000 Tetrahedron Lett. 41 89; (b) Kobayashi K, Shirasaka T, Sato A, Horn E and Furukawa N 1999 Angew. Chem. Int. Ed. 38 3483; (c) Sawaki T, Endo K, Kobayashi K, Hayashida O and Aoyama Y 1997 Bull. Chem. Soc. Jpn. 70 3075; (d) Kobayashi K, Kobayashi N, Ikuta M, Therrien B, Sakamoto S and Yamaguchi K 2005 J. Org. Chem. 70 749; (e) Tanaka T, Tasaki T and Aoyama Y 2002 J. Am. Chem. Soc. 124 12453; (f) Kobayashi K, Endo K, Aoyama Y and Masuda H 1993 Tetrahedron Lett. 34 7929
(a) Tominaga M, Katagiri K, and Azumaya I 2009 Cryst. Growth Des. 9 3692; (b) Tominaga M, Katagiri K and Azumaya I 2010 Cryst. Eng. Comm. 12 1164; (c) Tominaga M, Masu H and Azumaya I 2011 Cryst. Growth Des. 11 542; (d) Tominaga M, Masu H and Azumaya I 2011 Cryst. Eng. Comm. 13 5299
(a) Aitipamula S, Nangia A, Thaimattam R and Jaskolski M 2003 Acta Crystallogr. 39 481; (b) Venkataramanan B, Guan James W L, Vittal J J and Suresh V 2004 Cryst. Growth Des. 4 553; (c) Pigge F C, Dighe M K and Rath N P 2006 Cryst. Growth Des. 6 2732
Perrin D D, Armarego W L F and Perrin D R 1980 In Purification of Laboratory Chemicals (Oxford: Pergamon Press)
Ali A, Bansal D, Kaushik N K, Kaushik N, Choi E H and Gupta R 2014 J. Chem. Sci. 126 1091
SMART: Bruker Molecular Analysis Research Tool, version 5.618, Bruker Analytical X-ray System: Madison, WI, 2000
SAINT-NT, Version 6.04, Bruker Analytical X-ray System: Madison, WI, 2001
SHELXTL-NT, Version 6.10, Bruker Analytical X-ray System: Madison, WI, 2000
Altomare A, Cascarano G, Giacovazzo C and Guagliardi A 1993 J. Appl. Crystallogr. 26 343
Sheldrick G M 2008 Acta Crystallogr. Sec. A 64 112
Farrugia, L J WinGX version 1.64, An Integrated System of Windows Programs for the Solution, Refinement and Analysis of Single-Crystal X-ray Diffraction Data; Department of Chemistry, University of Glasgow, 2003
Spek A L, PLATON, A Multipurpose Crystallographic Tool, Utrecht University, The Netherlands, 2002
Geary W J 1971 Coord. Chem. Rev. 7 81
Mishra A, Kaushik N K, Verma A K and Gupta R 2008 Eur. J. Med. Chem. 43 2189
Mishra A, Ali A, Upreti S and Gupta R 2008 Inorg. Chem. 47 154
(a) Das A, Peng S –M, Lee G –H and Bhattacharya S 2004 New. J. Chem. 28 712; (b) Bag N, Lahiri G K, Bhattacharya S, Falvello LR and Chakravorty A 1988 Inorg. Chem. 27 4396; (c) Ellis R M, Quilligan J D, William N H and Yandell J K 1989 Aust. J. Chem. 42 1; (d) Barral M C, Aparicio R J, Royer E C, Saucedo M J, Urbanos F A, Puebla E G and Valero C R 1991 J. Chem. Soc., Dalton Trans. 1609
Acknowledgements
Authors thank Science and Engineering Research Board (SERB), Govt. of India for the generous financial support and CIF-USIC of this university for the instrumental facilities. AA and DB thank University Grant Commission (UGC) for their fellowships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Figures for absorption spectra, NMR spectra and thermal analysis; and tables for NMR spectra data. The electronic supplementary information can be seen at www.ias.ac.in/chemsci. CCDC 994231-994234 contain the crystallographic data for this paper. These data can be obtained free of charges from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
ALI, A., BANSAL, D. & GUPTA, R. Synthesis, characterization and self-assembly of Co3+ complexes appended with phenol and catechol groups. J Chem Sci 126, 1535–1546 (2014). https://doi.org/10.1007/s12039-014-0703-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0703-z