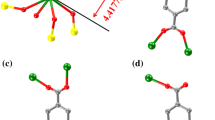
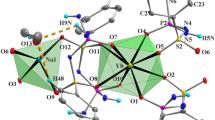
Abstract
One-dimensional isomorphous inorganic polymers containing Anderson type heteropoly anion as a basic building unit, namely [La(H2O)7Cr(OH)6Mo6O18]n⋅4nH2O (1), [Gd(H2O)7Cr(OH)6Mo6O18]n⋅4nH2O (2), [Gd(H2O)7Al(OH)6Mo6O18]n⋅4nH2O (3), and [Eu(H2O)7Al(OH)6Mo6O18]n⋅4nH2O (4) have been synthesized and studied by the powdered X–ray diffraction, TGA, IR, electronic and ESR spectroscopy, and unambiguously by single crystal X-ray crystallography. Isomorphous compounds 1-4 are crystallized in orthorhombic system with Pca2 1 space group. The crystal structure analysis reveals a one-dimensional extended chain in which the Anderson type heteropolyanion, acting as the building unit, is linked by rare earth metal ions in a zig-zag fashion. In the crystal structure, all types of oxygens of the heteropolyanion, lattice waters, lanthanum coordinated waters are extensively involved in O—H ⋯O hydrogen bonding interactions. Compounds are additionally characterized by UV-visible and ESR spectroscopy.

A series of four one-dimensional isomorphous inorganic solids namely, [La(H2O)7Cr(OH)6Mo6O18]n•4nH2O (1), [Gd(H2O)7Cr(OH)6Mo6O18]n•4nH2O (2), [Gd(H2O)7Al(OH)6Mo6O18]n•4nH2O (3), and [Eu(H2O)7Al(OH)6Mo6O18]n•4nH2O (4) have been synthesized and characterized by the powdered X–ray diffraction studies, TG analysis, IR-, electronic- and ESR-spectroscopy, and unambiguously by single crystal X˗ray crystallography.







Similar content being viewed by others
References
(a) Pope M T 1983 In Heteropoly and isopoly oxometalates, (Berlin: Springer-Verlag); (b) Pope MT and Müller A 1991 Angew. Chem. Int. Ed. Engl. 30 34; (c) Okumara T Mizuno N and Misono M 1996 Adv. Catal. 41 113; (d) Hill C L and Prosser-McCartha C M 1995 Coord. Chem. Rev. 143 407; (e) Arumuganathan T, Rao A S, Vijaykumar T and Das S K 2008 J. Chem. Sci. 120 95
Müller A, Peters F, Pope M T and Gatteschi D 1998 Chem. Rev. 98 239
Yamase T 1998 Chem. Rev. 98 307
Howell R C, Perez F G, Jain S, Horrocks W D and Francesconi L C 2001 Angew. Chem. Int. Ed. Engl. 41 4031
(a) Yamase T and Tomita K 1990 Inorg. Chim. Acta 169 147; (b) Yamase T and Sugeta M 1993 J. Chem. Soc. Dalton Trans. 759; (c) Khenkin A M and Neumann R 2002 Adv. Synth. Catal. 344 1017
(a) Peacock R D and Weakly T J R 1971 J. Chem. Soc (A) 1836; (b) Molchanov V N, Tatjanina I V, Torchenkova E A and Kazansky L P 1981 J. Chem. Soc. Chem. Comm. 93; (c) Yamase T, Naruke H and Sasaki Y 1990 J. Chem. Soc., Dalton Trans. 1687
Wassermann K and Pope M T 2001 Inorg. Chem.40 2763
Wu C-D, Lu C-Z, Zhuang H–H and Huang J–S 2002 J. Am. Chem. Soc. 124 3836
Wang X, Guo Y, Li Y, Wang E, Hu C and Hu N 2003 Inorg. Chem. 42 4135
Gaunt A J, May I, Sarsfield M J, Collision D, Helliwell M and Dennis I S 2003 J. Chem. Soc., Dalton Trans. 2767
Fukaya K and Yamase T 2003 Angew. Chem., Int. Ed. Engl. 42 654
(a) Naruke H, Ozeki T and Yamase T 1991 Acta. Crystallogr. C47 489; (b) Cronin L, Beugholt C, Krickemeyer E, Schmidtmann M, Bögge H, Kögerler P, Kim K, Luong K and Müller A 2002 Angew. Chem. Int. Ed. 41 2805
Sadkane M, Dickman M H and Pope M T 2000 Angew. Chem., Int. Ed. 39 2914
Shivaiah V, Reddy P V N and Das S K 2002 J. Chem. Soc. Dalton Trans. 3781
(a) Manikumari S, Shivaiah V and Das S K 2002 Inorg.Chem. 41 6953; (b) Shivaiah V Nagaraju M, Das S K 2003 Inorg. Chem. 42 6604; (c) Shivaiah V and Das S K 2005 J. Chem. Sci. 117 227
Perloff A 1970 Inorg. Chem. 9 2228
(a) Drewes D, Limanski E M and Krebs B 2004 J. Chem. Soc., Dalton Trans. 2087; (b) An H, Xiao D, Wang E, Sun C and Xu L 2005 Eur. J. Inorg. 854; (c) An H, Xiao D, Wang E and Xu L 2005 New J. Chem. 29 667; (d) An H, Lan Y, Wang E, Hao N, Xiao D, Duan L and Xu L 2004 Inorg. Chem. Commun. 7 356; (e) Drewes D and Krebs B 2005 Z. Anorg. Allg. Chem. 631 2591; (f) Liu F-X, Marchal-Roch C, Dambournet D, Acker A, Marrot J and Sécheresse F 2008 Eur. J. Inorg. Chem. 2191; (g) Li S, Ma P, Wang J, Guo Y, Niu H, Zhao J and Niu J 2010 CrystEngComm 12 1718; (h) Tan R-K, Liu S-X, Zhang W, Li S-J and Zhang Y-Y 2011 Inorg. Chem. Commun. 14 384; (i) Singh M, Lofland S E, Ramanujachary K V and Ramanan A 2010 Cryst. Growth Des. 10 5105
(a) An H, Xiao D, Wang E, Sun C and Lin X 2005 J. Mol. Structure 743 117; (b) An H, Han Z, Xu T, Meng, C and Wang E 2008 Inorg. Chem. Commun. 11 914; (c) Wang Y, Xiao D, Qi Y, Wang E and Liu J 2008 J. Clust. Sci. 19 367; (d) Li J, Zhang L-C, Sun Z-G, Tian C-H, Zhu Z-M, Zhao Y, Zhu Y-Y, Zhang J, Zhang N, Liu L and Lu X 2008 Z. Anorg. Allg. Chem. 634 1173; (e) Yang, Y, Xu L, Li F and Qu X 2013 Inorg. Chem. Commun. 33 142
Andreev G B, Fedoseev A M, Budantseva N A and Antipin M Yu 2002 Crystallography Reports 47 394
Fedoseev A M, Budantseva N A, Andreev G B, Yusov A B, and Shirokova I B 2002 Russ. J. Coord. Chem. 28 279
Shi D-M, Ma F-X, Zhang C-J, Lu S and Chen Y-G 2008 Z. Anorg. Allg. Chem. 634 758
Sheldrick G M 1996 SADABS, A program for absorption correction with the Siemens SMART area-detector system (Germany: University of Göttingen)
Sheldrick G M 1997 SHELXS-97, A program for solution of crystal structures(Germany: University of Göttingen)
Sheldrick G M 1997 SHELXL-97, A program for solution and refinement of crystal structures (Germany: University of Göttingen)
Hall R D 1907 J. Am. Chem. Soc. 29 692
(a) Golhen S, Ouahab L, Grandjean D and Molinie P 1998 Inorg. Chem. 37 1499; (b) Ouahab L, Golhen S, Yoshida Y and Saito G 2003 J. Cluster Sci. 14 193
Acknowledgements
We thank Science & Engineering Research Board (a statutory body under the Department of Science and Technology), Government of India, for financial support (Project No. SB/SI/IC/034/2013). The national X-ray diffractometer facility at University of Hyderabad, by the Department of Science and Technology, Government of India is gratefully acknowledged. TA and SR are grateful to the Council of Scientific and Industrial Research (CSIR), Government of India, for fellowships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
FT-IR spectra and powder X-ray diffraction patterns for compounds 1-4, TGA plots for compounds 1, 3 and 4 and some figures related to the crystal structures are available at
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SHIVAIAH, V., CHATTERJEE, T. & DAS, S.K. Coordination of lanthanide cation to an Anderson type polyoxometalate anion leads to isomorphous metal-oxide based one-dimensional inorganic solids: Synthesis, crystal structure and spectroscopy. J Chem Sci 126, 1525–1533 (2014). https://doi.org/10.1007/s12039-014-0688-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0688-7