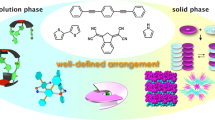
Abstract
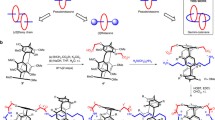
A modular design of a molecular tweezer is presented that integrates a multipolar D- π-A [D: Donor, A: Acceptor] scaffold, 1-aminopyrene-based fluorophore units and L-alanine-based linkers. The synthesis of the molecule is based on two-fold aromatic nucleophilic reactions (ArSN) and coupling reactions of the acid and amino functionalities. This molecule crystallizes in a non-centrosymmteric (P21) space group. We present its rich self-assembly characteristics that involves an array of π-stacking interactions. In addition, the molecular tweezer within its cleft forms H-bonding with two dimethylformamide molecules. Such multipolar D- π-A systems containing chiral and fluorophore units are potential candidates for a number of electronic and photonic applications.

Self-assembly of a multipolar scaffold having chiral units and flurophores was observed and characterized.






Similar content being viewed by others
References
(a) Hoeben F J M, Jonkheijm P, Meijer E W and Schenning A P H J 2005 Chem. Rev. 105 1491; (b) Zang L, Che Y and Moore J S 2008 Acc. Chem. Res. 41 1596
(a) Wang L, Li W, Lu J, Zhao Y-X, Fan G, Zhang J-P and Wang H 2013 J. Phys. Chem. C 117 26811; (b) Ding L, Liu Y, Cao Y, Wang L, Xin Y and Fang Y 2012 J. Mater. Chem. 22 11574; (c) Nishizawa S, Kato Y and Teramae N 1999 J. Am. Chem. Soc. 121 9463; (d) Wang M, Xu J, Liu X and Wang H 2013 New J. Chem. 37 3869; (e) Ingale S A and Seela F 2012 J. Org. Chem. 77 9352; (f) Zhou Y, Zhu C-Y, Gao X-S, You X-Y and Yao C 2010 Org. Lett. 12 2566; (g) Cho H, Lee S, Cho N S, Jabbour G E, Kwak J, Hwang D-H and Lee C 2013 ACS Appl. Mater. Interfaces 5 3855; (h) Zhang H, Wang Y, Shao K, Liu Y, Chen S, Qiu W, Sun X, Qi T, Ma Y, Yu G, Su Z and Zhu D 2006 Chem. Commun. 755; (i) Wu N-W, Zhang J, Ciren D, Han Q, Chen L-J, Xu L and Yang H-B 2013 Organometallics 32 2536
Figueira-Duarte T M and Müllen K 2011 Chem. Rev. 111 7260
(a) Vybornyi M, Rudnev A V, Langenegger S M, Wandlowski T, Calzaferri G and Häner R 2013 Angew. Chem. Int. Ed. 52 11488; (b) Wang M, Xu J, Liu X and Wang H 2013 New J. Chem. 37 3869; (c) Tang H, Oliveira C S de, Sonntag G, Gibb C L D, Gibb B C and Bohne C 2012 J. Am. Chem. Soc. 134 5544; (d) Li W, Wang L, Zhang J-P and Wang H 2014 J. Mater. Chem. C 2 1887
(a) Winnik F M 1993 Chem. Rev. 93 587; (b) Goedeweeck M, Auweraer M V der and Schryver F C De 1985 J. Am. Chem. Soc. 107 2334; (c) Song X and Swanson B I 1999 Langmuir 15 4710; (d) Sahoo D, Narayanaswamy V, Kay C M and Ryan R O 2000 Biochemistry 39 6594; (e) Lewis F D, Zhang Y and Letsinger R L 1997 J. Am. Chem. Soc. 119 5451
(a) Chen Z, Lohr A, Saha-Möller C R and Würthner F 2009 Chem. Soc. Rev. 38 564; (b) Zhang L, Liu C, Jin Q, Zhu X and Liu M 2013 Soft Matter 9 7966
(a) Ajayakumar M R, Hundal G and Mukhopadhyay P 2013 Chem. Commun. 49 7684; (b) Ajayakumar M R, Asthana D and Mukhopadhyay P 2012 Org. Lett. 14 4822; (c) Ajayakumar M R and Mukhopadhyay P 2010 Org. Lett. 12 2646; (d) Ajayakumar M R and Mukhopadhyay P 2009 Chem. Commun. 3702
(a) Asthana D, Pandey R and Mukhopadhyay P 2013 Chem. Commun. 49 451; (b) Mukhopadhyay P, Bharadwaj P K, Krishnan A and Das P K 2002 J. Mater. Chem. 12 2786; (c) Mukhopadhyay P, Bharadwaj P K, Savitha G, Krishnan A and Das P K 2002 J. Mater. Chem. 12 2237; (d) Mukhopadhyay P, Bharadwaj P K, Savitha G, Krishnan A and Das P K 2000 Chem. Commun. 1815
Asthana D, Kumar A, Pathak A, Sukul P K, Malik S, Chatterjee R, Patnaik S, Rissanen K and Mukhopadhyay P 2011 Chem. Commun. 47 8928
(a) Lehn J-M 1995 In Supramolecular Chemistry: Concepts and Perspectives (Weinheim: Wiley-VCH) p 1-271; (b) Desiraju G R 1996 In Perspectives in Supramolecular Chemistry: The Crystal as a Supramolecular Entity Vol 2 (Chichester: Wiley)
Feuer H, Bachmann G B and Kispersky J P 1951 J. Am. Chem. Soc. 73 3575
(a) Martínez-Mán\(\tilde {\textnormal {e}}\)z R and Sancenón F 2003 Chem. Rev. 103 4419; (b) Kim S K, Bok J H, Bartsch R A, Lee J Y and Kim J S 2005 Org. Lett. 7 4839; (c) Liao J-H, Chen C-T and Fang J-M 2002 Org. Lett. 4 561; (d) Wang L, Li W, Lu J, Zhang J-P, Wang H 2014 Tetrahedron 70 3172; (e) Kim H J, Kim S K, Lee J Y and Kim J S 2006 J. Org. Chem. 71 6611; (f) Lu H, Wang Q, Li Z, Lai G, Jiang J and Shen Z 2011 Org. Biomol. Chem. 9 4558; (g) Ziessel R, Ulrich G, Haefele A and Harriman A 2013 J. Am. Chem. Soc. 135 11330; (h) Zaragoza-Galán G, Fowler M, Rein R, Solladiá N, Duhamel J and Rivera E 2014 J. Phys. Chem. C 118 8280
(a) Kaafarani B R, El-Ballouli A O, Trattnig R, Fonari A, Sax S, Wex B, Risko C, Khnayzer R S, Barlow S, Patra D, Timofeeva T V, List E J W, Brédase J-L and Marder S R 2013 J. Mater. Chem. C 1 1638; (b) Bains G K, Kim S H, Sorin E J and Narayanaswami V 2012 Biochemistry 51 6207; (c) Zhang L, Liu C, Jin Q, Zhua X and Liu M 2013 Soft Matter 9 7966; (d) Ding L, Liu Y, Cao Y, Wang L, Xin Y and Fang Y 2012 J. Mater. Chem. 22 11574; (e) Aguilar-Martínez M, Bautista-Martínez J A and Rivera E 2008 Designed Monomers and Polymers 11 173; (f) Liu X-T, Zhao Y, Ren A-M and Feng J-K 2011 J. Mol. Model. 17 1413; (g) Nakamura M, Fukunaga Y, Sasa K, Ohtoshi Y, Kanaori K, Hayashi H, Nakano H and Yamana K 2005 Nucleic Acids Res. 33 5887
(a) Birks J B 1970 In Photophysics of aromatic molecules (New York: Wiley-Interscience); (b) Birks J B and Christophorou L G 1963 Spectrochimica Acta 19 401; (c) Smalley M K and Silverman S K 2006 Nucleic Acids Res. 34 152; (d) Li M-C, Ho R-M and Lee Y-D 2011 J. Mater. Chem. 21 2451; (e) Nakamura M, Fukuda M, Takada T and Yamana K 2012 Org. Biomol. Chem. 10 9620
(a) Klapper H and Hahn Th 2002 Point Group Symmetry and Physical Properties of Crystals: International Tables for Crystallography A 804; (b) Braga D, Grepioni F and Desiraju G R 1998 Chem. Rev. 98 1375; (c) Halasyamani P S and Poeppelmeier K R 1998 Chem. Mater. 10 2753
(a) Sagara Y, Mutai T, Yoshikawa I and Araki K 2007 J. Am. Chem. Soc. 129 1520; (b) Zhang S, Qiao X, Chen Y, Wang Y, Edkins R M, Liu Z, Li H and Fang Q 2014 Org. Lett. 16 342; (c) Morales-Espinoza E G, Lijanova I V, Morales-Saavedra O G, Torres-Zuñiga V, Hernandez-Ortega S and Martínez-García M 2011 Molecules 16 6950; (d) Morales-Saavedra O G and Rivera E 2006 Polymer 47 5330
(a) Dong J, Wang Y, Zhang J, Zhan X, Zhu S, Yang H and Wang G 2013 Soft Matter 9 370; (b) Li W, Wang L, Zhang J-P and Wang H 2014 J. Mater. Chem. C 2 1887; (c) Etika K C, Jochum F D, Cox M A, Schattling P, Theato P and Grunlan J C 2010 Macromolecules 43 9447
Acknowledgements
We acknowledge DBT BUILDER, DST PURSE for the funding and the central instrumentation facility at AIRF, JNU for IR, Mass and NMR studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
The detailed procedure for synthesis and characterization, MALDITOF Mass, IR, 1H and 13C, DEPT-135, APT NMR spectra, X-ray crystallographic tables are given in SI file available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
ASTHANA, D., HUNDAL, G. & MUKHOPADHYAY, P. Self-assembly characteristics of a multipolar donor-acceptor–based bis-pyrene integrated molecular tweezer. J Chem Sci 126, 1331–1336 (2014). https://doi.org/10.1007/s12039-014-0687-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0687-8