Abstract
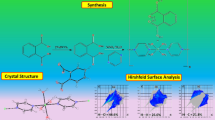
Highly flexible Zn(II)1,2-bis(meso-octaethylporphyrin)ethane (1) has been used as host in which two porphyrin rings are found to be face-to-face in non-coordinating solvents. Upon addition of one relatively smaller 4,4′-dipyridine (L1) and one extended N,N′-bispyridine-4-yl-methylene ethylenediamine (L2) guest ligands, the syn conformation of 1 is switched to the anti complexes 1∙(L1)2 and 1∙L 2, respectively. Single crystal X-ray structures of all the complexes are reported in which a stable one-dimensional coordination polymer is produced only in 1∙L 2 that is, to the best of our knowledge, the first structural report of 1D-coordination polymer with porphyrin dimer. Solution structures of the complexes along with binding studies in solution between 1 and L have also been investigated. The morphology of the polymeric complex 1∙L 2 on silicon wafer surface was examined by Atomic Force Microscopy (AFM) in which the crystalline islands of well defined facets of size ranging from 200–550 nm perimeter and a height of 20–40 nm have been observed.

Highly flexible Zn(II)bisporphyrin, which is found to be cofacial, forms a stable 1D coordination polymer with N,N′-bispyridine-4-yl-methylene ethylenediamine which, on silicon wafer surface, produces crystalline islands of well defined facets of size ranging from 200–550 nm perimeter and a height of 20-40 nm.














Similar content being viewed by others
References
Liu C Y and Bard A J 2002 Nature 418 162
Tsuda A and Osuka A 2001 Science 293 79
(a) Beletskaya I, Tyurin V S, Tsivadze A Y, Guilard R and Stern C 2009 Chem. Rev. 109 1659; (b) Aratani N, Kim D and Osuka A 2009 Acc. Chem. Res 42 1922; (c) Maeda C, Kamada T, Aratani N and Osuka A 2007 Coord. Chem. Rev 251 2743; (d) Iengo E, Zangrando E and Alessio E 2006 Acc. Chem. Res 39 841
(a) Monti D, Nardis S, Stefanelli M, Paolesse R, Natale C D and Amico A D 2009 J. Sensors 1; (b) Sanders J K M 2000 In The Porphyrin Handbook Kadish K M, Smith K M and Guilard R (Eds.) New York: Academic Press 3 347
(a) Michelsen U and Hunter C A 2000 Angew. Chem. Int. Ed. 39 764; (b) Ogawa K and Kobuke Y 2000 Angew. Chem. Int., Ed. 39 4070; (c) Ikeda C, Fujiwara E, Satake A and Kobuke Y 2003 Chem. Commun. 616; (d) Yoon D H, Lee S B, Yoo K H, Kim J, Lim J K, Aratani N, Tsuda A, Osuka A and Kim D 2003 J. Am. Chem. Soc. 125 11062
(a) Choi M S, Yamazaki T, Yamazaki I and Aida T 2004 Angew. Chem. Int. Ed. 43 150; (b) Holten D, Bocian D F and Lindsey J S 2002 Acc. Chem. Res. 35 57; (c) Burrell A K, Officer D L, Plieger P G and Reid D C W 2001 Chem. Rev. 101 2751
(a) Tanaka T, Lee B S, Aratani N, Yoon M -C, Kim D and Osuka A 2011 Chem. Eur. J. 17 14400; (b) Song J, Aratani N, Kim P, Kim D, Shinokubo H and Osuka A 2010 Angew. Chem. Int. Ed. 49 3617; (c) Song J, Aratani N, Shinokubo H and Osuka A 2010 Chem. Eur. J. 16 13320; (d) Hori T, Peng X, Aratani N, Takagi A, Matsumoto T, Kawai T, Yoon Z S, Yoon M C, Yang J, Kim D and Osuka A 2008 Chem. Eur. J. 14 582; (e) Hori T, Aratani N, Takagi A, Matsumoto T, Kawai T, Yoon M. -C, Yoon Z. S, Cho S, Kim D and Osuka A 2006 Chem. Eur. J. 12 1319; (f) Maes W, Vanderhaeghen J, Smeets S, Asokan C V, Renterghem L M V, Prez F E D, Smet M and Dehaen W 2006 J. Org. Chem. 71 2987
(a) Satake A, Azuma S, Kuramochi Y, Hirota S and Kobuke Y 2011 Chem. Eur. J 17855; (b) Kuramochi Y, Sandanayaka A S D, Satake A, Araki Y, Ogawa K, Ito O and Kobuke Y 2009 Chem. Eur. J 15 2317; (c) Kuramochi Y, Satake A, Itou M, Ogawa K, Araki Y, Ito Y and Kobuke Y 2008 Chem. Eur. J 14 2827; (d) Dy J T, Ogawa K, Satake A, Ishizumi A and Kobuke Y 2007 Chem. Eur. J. 13 3491; (e) Diskin-Posner Y, Patra G K and Goldberg I 2001 Dalton Trans 2775; (g) Kumar D K, Das A and Dastidar P 2007 Inorg. Chem 46 7351
(a) Hasobe T 2010 Phys. Chem. Chem. Phys. 12 44; (b) Medforth C J, Wang Z, Martin K E, Song Y, Jacobsenc J L and Shelnutt J A 2009 Chem. Commun. 7261; (c) Hunter C A and Tomas S 2006 J. Am. Chem. Soc 128 8975; (d) Stulz E, Scott S M, Ng Y-F, Bond A D, Teat S J, Darling S L, Feeder N and Sanders J K M 2003 Inorg. Chem. 42 6564; (e) Aratani N, Osuka A, Kim Y H, Jeong D H and Kim D 2000 Angew. Chem, Int. Ed. 39 1458
Satake A, Fujita M, Kurimotoa Y and Kobuke Y 2009 Chem. Commun 1231
(a) Jordan P, Fromme P, Witt H T, Klukas O, Saenger W and Krauss N 2001 Nature 411 909; (b) McDermott G, Prince S M, Freer A A, Lawless A M H, Papiz M Z, Cogdell R J and Isaacs N W 1995 Nature 374 517; (c) Deisenhofer J and Michel H 1989 Science 245 1463; (d) Allen J P, Feher G, Yeates T O, Rees D C, Deisenhofer J, Michel H and Huber R 1986 Proc. Natl. Acad. Sci. 83 8589; (e) Deisenhofer J, Epp O, Miki K, Huber R and Michel H 1985 Nature 318 618
(a) Ikbal S A, Brahma S and Rath S P 2012 Inorg.Chem. 51 9666; (b) Dey S, Ikbal S A and Rath S P 2014 New J. Chem. 38 1458
(a) Borovkov V V and Inoue Y 2009 Eur. J. Org. Chem. 189; (b) Borovkov V V, Lintuluoto J M, Sugeta H, Fujiki M, Arakawa R and Inoue Y 2002 J. Am. Chem. Soc. 124 2993; (c) Borovkov V V, Lintuluoto J M, Hembury G A, Sugiura M, Arakawa R, Inoue Y 2003 J. Org. Chem. 68 7176; (d) Fujii I, Borovkov V V and Inoue Y 2006 Anal. Sci. 22X77; (e) Borovkov V V, Fujii I, Muranaka A, Hembury G A, Tanaka T, Ceulemans A, Kobayashi N and Inoue Y 2004 Angew.Chem.Int.Ed. 43 5481
Kennedy A R, Brown K G, Graham D, Kirkhouse J B, Kittner M, Major C, McHugh C J, Murdoch P and Smith W E 2005 New J. Chem. 29 826
SAINT + , 6.02 ed. Bruker AXS, Madison, WI, 1999
Sheldrick G M 2000 SADABS 2.0
Sheldrick G M 2008 Acta Cryst.A 64 112
Spek A L 2003 J. Appl. Crystallogr. 36 7
(a) Brahma S, Ikbal S A and Rath S P 2011 Inorg. Chim. Acta. 372 62; (b) Chaudhary A, Ikbal S A, Brahma S and Rath S P 2013 Polyhedron 52 761; (c) Brahma S, Ikbal S A and Rath S P 2014 Inorg. Chem. 53 62; (d) Brahma S,Ikbal S A, Dhamija A and Rath S P 2014 Inorg. Chem. 53 2381; (e) Dey S, Rath S P 2014 Dalton Trans. 43 2301; (f) Brahma S, Ikbal S A, Dey S and Rath S P 2014 Chem. Commun. 48 4070
Kasha M, Rawls H R and Bayoumi M A E 1965 Pure Appl. Chem. 11 371
(a) Davidson G J E, Lane L A, Raithby P R, Warren J E, Robinson C V and Sanders J K M 2008 Inorg. Chem. 47 8721; (b) Plieger P, Burrell A K, Hall S B and Officer D L J 2005 Inclusion Phenom. Macrocyclic Chem. 53 143
Twyman L J and King A S H 2002 Chem Commun 910
(a) www.hyperquad.co.uk/HypSpec.htm.; (b) Gans P, Sabatini A and Vacca A 1996 Talanta 43 1739
(a) Hillier A C and Ward M D 1994 Science 263 1261; (b) Evans C G , Hariadi R F and Winfree E 2012 J. Am. Chem. Soc. 134 10485
Acknowledgement
We are thankful to the Science & Engineering Research Board (SERB) and Council of Scientific and Industrial Research (CSIR), New Delhi for financial support. S.A.I., S.B. and A.D. thank CSIR, India for their fellowships. We thank Prof. Thiruvancheril G. Gopakumar for valuable discussion regarding AFM measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
CCDC-999047 and 999048 contain the X-ray crystallographic details in CIF format for compound 1∙(L1)2 and 1∙ L2, respectively. These data can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.htmlor [from the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: + 44 1223 336 033; E-mail: deposit@ccdc.cam.ac.uk].
Electronic supplementary material
Rights and permissions
About this article
Cite this article
IKBAL, S.K.A., BRAHMA, S., DHAMIJA, A. et al. Building-up novel coordination polymer with Zn(II) porphyrin dimer: Synthesis, structures, surface morphology and effect of axial ligands. J Chem Sci 126, 1451–1461 (2014). https://doi.org/10.1007/s12039-014-0686-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0686-9