Abstract
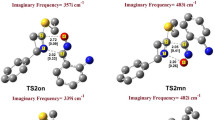
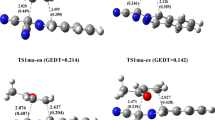
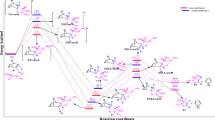
A theoretical study of the regio- and stereoselectivities of the 1,3-dipolar cycloaddition reaction between methyl crotonate and pyrroline-1-oxide has been carried out using density functional theory (DFT) at the B3LYP/6-31G(d) level of theory. The reaction has been followed by performing transition state optimization, calculations of intrinsic reaction coordinate and activation energies; the molecular mechanism of the reactions is concerted and asynchronous. The regio- and exo/endo-selectivity have been explained in terms of frontier molecular orbital interactions, local and global electrophilicity and nucleophilicity indices and an analysis of the Wiberg bond indices in the transition state. The FMO analysis and DFT-based reactivity indices showed that the regioselectivity of this reaction is controlled by the HOMOdipole–LUMOdipolarophile interaction. The activation parameters indicated favoured endo approach along the meta-pathway in agreement with the experimental results.

The molecular mechanism of the 1,3-dipolar cycloaddition reaction between pyrroline-1-oxide and methyl crotonate is theoretically investigated by DFT method at the B3LYP/6-31G* level. The transition states corresponding to the possible stereoisomeric pathways along regioisomeric reaction channels were searched, localized and optimized. The cycloaddition is favored along the reaction channel with the meta-endo adduct as major diastereomer in agreement with experiment.






Similar content being viewed by others
References
Padw A 1984 1,3-Dipolar cycloaddition chemistry, vols 1-2 (New York: Wiley Interscience)
(a) Padwa A, Tomioka Y and Venkatramanan MK 1987 Tetrahedron Lett. 28 755; (b) Breuer E, Aurich H G and Nielsen 1989 Nitrones, nitronates and nitroxides (New York: Wiley); (c) Padwa A 1991 Comprehensive Organic Synthesis 4 1069; (d) Torssell K B G 1998 Nitrile oxides, nitrones and nitronates in organic synthesis; (New York: VCH); (e) Gothelf K V and Jørgensen K A 1998 Chem. Rev. 98 863; (f) Bortolini O, Mulani I, De Nin A, Maiuolo L, Nard M, Russo B and Avnet S 2011 Tetrahedron 67 563; (g) Majumder S and Bhuyan P J 2012 Tetrahedron Lett. 53 762
(a) Minter A R, Brennan B B and Mapp A K 2004 J. Am. Chem. Soc. 126 10504; (b) Palmer G C, Ordy M J, Simmons R D, Strand J C, Radov L A, Mullen G, Kinsolving C R, Mitchell J T and Alle S D 1989 Antimicrob. Agents Chemother. 33 895
Ding P, Miller M, Chen Y, Helquist P, Oliver A J and Wiest O 2004 Org. Lett. 6 1805
Wess G, Kramer W, Schuber G, Enhsen A, Baringhaus K H, Globmik H, Müller S, Bock K, Klein H, John M, Neckermann G and Hoffmann A 1993 Tetrahedron Lett. 34 819
Padwa A and Pearson W H 2002 Synthetic applications of 1,3-dipolar cycloaddition chemistry toward heterocycles and natural products (New York: Wiley & Sons)
Marakchi K, Kabbaj O K and Komiha N 2002 J. Fluorine Chem. 114 81
Marakchi K, Kabbaj O K, Komiha N, Jalal R and Esseffar M 2003 J. Mol. Struct. (Theochem) 620 271
Liu J, Niwayama S, You Y and Houk K N 1998 J. Org. Chem. 63 1064
Cossó F P, Marao I, Jiao H and Schleyer P V R 1999 J. Am. Chem. Soc. 121 6737
Cadra V, Portoleś R, Murga J, Uriel S, Marco J A, Domingo L R and Zaragoza R J 2000 J. Org. Chem. 65 7000
Domingo L R 2000 Eur. J. Org. Chem. 2265
Nacereddine A K, Yahia W, Bouacha S and Djerourou A 2010 Tetrahedron Lett. 51 2617
Stecko S, Michel C, Milet A, Pérez S and Chmielewski M 2008 Tetrahedron: Asymmetry 19 2140
Acharjee N and Banerji A 2011 Comput. Theor. Chem. 967 50
Asrof Ali Sk, Khan J H, Wazeer M I M and Perzanowski H P 1989 Tetrahedron 45 5979
Frisch M J et al. Gaussian 03, Revision B.04 Gaussian: Pittsburgh PA 2003
Becke A D J 1988 Chem. Phys. 38 3098
Lee C Yang and W Parr R G 1988 Phys. Rev. B37 785
Hehre W J, Radom L, Schleyer P R and Pople J A 1986 Ab initio molecular orbital theory (New York: Wiley)
Gonzalez C and Schlegel H B 1989 J. Chem. Phys. 90 2154
Gonzalez C and Schlegel H B 1990 J. Phys. Chem. 94 5523
(a) Reed A E, Curtiss L A and Weinhold F 1988 Chem. Rev. 88 899; (b) Reed A E Weinstock R B and Weinhold F 1985 J. Chem. Phys. 83 735
Parr R G and Yang W 1989 Density functional theory of atoms and molecules (New York: Oxford University)
(a) Domingo L R, Chamorro E and Pérez P J 2088 J. Org. Chem. 73 4615; (b) Domingo L R and Picher M T 2004 Tetrahedron 60 5053
(a) Domingo L R, Aurell M J, Pérez P and Contreras R 2002 J. Phys. Chem. A106 6871
Geerlings P, De Prof F and Langenaeker W 2003 Chem. Rev. 103 1793
Pérez P, Domingo L R, Duque-Norna M and Chamorro E 2009 J. Mol. Struct. (Theochem) 895 86
Yang W and Mortier W J 1986 J. Am. Chem. Soc. 108 5708
Tomasi J and Persico M 1994 Chem. Rev. 94 2027
Kumar Das T, Salampuria S and Banerjee M 2010 J. Mol. Struct. (Theochem) 959 22
Ohgaki E, Motoyoshiya J, Narita S, Kakurai T, Hayashi S and Hirakawa K 1990 J. Chem. Soc. Perkin Trans. 1 3109
Aso M, Ojida A, Yang G, Cha O, Osawa E and Kanematsu K 1993 J. Org. Chem. 58 3960
Steck S, Michel C, Milet A, Perez S and Chmielewski M 2008 Tetrahedron: Asymmetry 19 1660
Wei D, Zhu Y, Zhang C, Sun D, Zhang W and Tang M 2011 J. Mol. Catal. A: Chem. 334 108
Marakchi K, Kabbaj O K, Komiha N and Chraibi M 2001 J. Fluorine Chem. 109 163
Marakchi K, Kabbaj O K, Komiha N and Abou El Makarim H 2010 Phys. Chem. News 52 128
Naji N, Marakchi K, Kabbaj O K, Komiha N, Chraibi M, Joffre J and Soufiaoui M 1999 J. Fluorine Chem. 94 127
Sheng Y H, Fang D C, Wuc Y D, Fub X Y and Jianga Y 1999 J. Mol. Struct. (Theochem) 467 31
Sustman R and Sicking W 1987 Chem. Ber. 120 1653
Sustman R and Sicking W 1987 Chem. Ber. 120 1471
Houk K N 1975 Acc. Chem. Res. 8 361
Merino P, Revuelta J, Tejero T, Chiacchio U, Rescifina A and Romeo G 2003 Tetrahedron 59 3581
Pérez P, Domingo L R, Aurell M J and Conteras R 2003 Tetrahedron 59 3117
(a) Domingo L R, Aurell M J, Pérez P and Contreras R 2002 Tetrahedron 58 4417; (b) Domingo L R, Asensio A and Arroyo P 2002 J. Phys. Org. Chem. 15 660; (c) Domingo L R, Arno M, Contreras R and Pérez P 2002 J. Phys. Chem. A 106 952; (d) Domingo LR 2002 Tetrahedron 58 3765; (e) Domingo L R, Aurell M J, Pérez P and Contreras R 2003 J. Org. Chem. 68 3884; (f) Domingo L R and Andres J 2003 J. Org. Chem. 68 8662; (g) Jasiński R, Koifman O I and Barański A 2011 Mendeleev Commun. 21 262; (h) Domingo L R, Pérez P and Sáez J A 2004 Tetrahedron 60 11503
Simkin B Y and Sheikhet I I 1995 Quantum chemical and statistical theory of solutions. A computational approach (London: Ellis Horwood Ltd.)
(a) Cances M T, Mennunci V and Tomasi J 1997 J. Chem. Phys. 107 3032; (b) Cossi M, Barone V, Cammi R and Tomasi J 1996 J. Chem. Phys. Lett. 255 327; (c) Barone V, Cossi M and Tomasi J 1998 J. Comput. Chem. 19 404
Mendez F, Tamariz J and Geerlings P 1998 J. Phys. Chem. A102 6292
Froese R D J, Organ M G, Goddard J D, Stack T D P and Trost B M 1995 J. Am. Chem. Soc. 117 10931
Garcia J I, Martinez-Merino V, Mayoral J A and Salvatella L 1998 J. Am. Chem. Soc. 120 2415
Domingo L R 1999 J. Org. Chem. 64 3922
Wiberg K B 1968 Tetrahedron 24 1083
Acknowledgement
This work was supported by the Ministry of High Education of Morocco (SCH09/09) project ‘Plan d’Urgence’ and European PF7 Marie Curie PIRSES-GA-2012-317544 project CAPZEO.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MARAKCHI, K., GHAILANE, R., KABBAJ, O.K. et al. DFT study of the mechanism and stereoselectivity of the 1,3-dipolar cycloaddition between pyrroline-1-oxide and methyl crotonate. J Chem Sci 126, 283–292 (2014). https://doi.org/10.1007/s12039-013-0563-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0563-y