Abstract
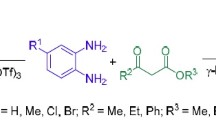
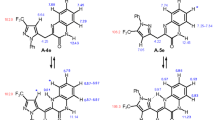
An exclusive regioselective formation of 2-thiomethyl ether substituted isomer of the privileged nucleus of 1,5-benzodiazepines was achieved from the reaction of p-nitro-o-phenylenediamine with a variety of α-oxoketene dithioacetals derived from several active methylene compounds, by exploiting the strategy based on the variation of electrophilicity of the two electrophilic centers of α-oxoketene dithioacetals and the hard and soft nucleophilic profiles of the p-nitro substituted o-phenylenediamines.

An exclusive regioselective formation of 2-thiomethyl ether substituted isomer of the privileged nucleus of 1,5-benzodiazepines was achieved from the reaction of p-nitro-o-phenylenediamine with a variety of α-oxoketene dithioacetals derived from several active methylene compounds.


Similar content being viewed by others
References
Chadha S, Paul S and Kapoor K K 2011 J. Chem. Pharm. Res. 3(2) 331
Wang Z X and Qin H L 2005 J. Hetrocycl. Chem. 42(5) 1001
Wei X L, Min Z and Hua S W 2011 Chem. Res. Chinese Universities 27 228
Kuo C W, Wang C C, Kavala V and Yao C F 2008 Molecules 13(9) 2313
(a) Kumar R and Joshi Y C 2007 Arkivoc 13 142; (b) Kumar R and Joshi Y C 2008 J. Serb. Chem. Soc. 73(10) 937; (c) Parveen A, Patil V A, Baseer M A and Ahmed S K 2011 Int. J. Ind. Chem. 2(3) 144
Yadav K M, Mohanta P K, lla H and Junjappa H 1996 Tetrahedron 52 14049
Junjappa H, Ila H and Asokan C V 1990 Tetrahedron 46(16) 5423
Mang W, Shao-Gaung S and Qun L 2004 Synth. Commun. 34(2) 287
(a) Ali S M and Tanimoto S 1990 Bull. Inst. Chem. Res. 68(3) 199; (b) Marino J P and Kostusyk J L 1979 Tetrahedron Lett. 20(27) 2489; (c) Chauhan S M S and Junjappa H 1976 Tetrahedron 32(14) 1779
Acknowledgements
Authors are thankful to the Punjab University, Chandigarh for providing the spectral data of the compounds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
CHAUDHARY, P., GUPTA, A., DEVI, P. et al. Hard and soft electrophilic and nucleophilic dissymmetry of α-oxoketenedithioacetals and p-nitro-o-phenylenediamine exploited to achieve the regioselectivity in the synthesis of 2-thiomethyl ether substituted isomer of the privileged nucleus of 1,5-benzodiazepines over to its 4-substituted isomers. J Chem Sci 125, 1487–1491 (2013). https://doi.org/10.1007/s12039-013-0520-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0520-9