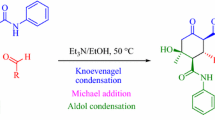
Abstract
Highly chemoselective environment friendly deprotection of acetonides and cleavage of acetals and ketones has been achieved by heating in aqueous medium without using any catalyst and organic solvent.

A chemoselective environment friendly methodology has been developed for deprotection of acetonides and cleavage of acetals and ketones in aqueous medium without using any catalyst and organic solvent.

Similar content being viewed by others
References
(a) Schkeryantz J M and Danishefsky S J 1995 J. Am. Chem. Soc. 117 4722; (b) Masters J J, Link J T, Snyder L B, Young W B and Danishefsky S J 1995 Angew. Chem., Int. Ed. Engl. 34 1723; (c) Boyce R J and Pattenden G 1996 Tetrahedron Lett. 37 3501
(a) Clode D M 1979 Chem. Rev. 79 491; (b) Nicolaou K C, Daines R A, Uenishi J, Li W S, Papahatjis D P and Chakraborty T K 1988 J. Am. Chem. Soc. 110 4672
(a) Banerjee S and Ghosh S J 2003 Org. Chem. 68 3981; (b) Krishna P R, Kannan V and Sharma G V M 2004 J. Org. Chem. 69 6467; (c) Hanessian S, Huang G, Chenel C, Machaalani R and Loiseleur O 2005 J. Org. Chem. 70 6721; (d) Sharma G V M and Gopinath T 2005 Tetrahedron Lett. 46 1307
(a) Fleet G W J and Smith P W 1985 Tetrahedron Lett. 26 1469; (b) Gerspacher M and Rapoport H 1991 J. Org. Chem. 56 3700; (c) Yadav J S, Chander M C and Reddy K K 1992 Tetrahedron Lett. 33 135; (d) Manna S, Viala J, Yadagiri P and Falck J R 1986 Tetrahedron Lett. 27 2679; (e) Park K H, Yoon Y J and Lee S G 1994 Tetrahedron Lett. 35 9737; (f) Leblanc Y, Fitzsimmons B J, Adams J, Perez F and Rokach J 1986 J. Org. Chem. 51 789; (g) Baurle S, Hoppen S and Koert U 1999 Angew. Chem., Int. Ed. 38 1263; (h) Ichihara A, Ubukata M and Sakamura S 1997 Tetrahedron Lett. 38 3473
(a) Barbot F and Miginiac P 1983 Synthesis 651; (b) Sterzycki R 1979 Synthesis 724; (c) Kantam M L, Swapna V and Santhi P L 1995 Synth. Commun. 25 2529; (d) Xiao X and Bai D 2001 Synlett 4, 535; (e) Vijayasaradhi S, Singh J and Aidhen I S 2000 Synlett 1 110; (f) Kim K S, Song Y H, Lee B H and Hahn C S 1986 J. Org. Chem. 51 404; (g) Yadav J S, Raghavendra S, Satyanarayana M and Balanarsaiah E 2005 Synlett 16 2461; (h) Swamy N R and Venkateswarlu Y 2002 Tetrahedron Lett. 43 7549; (i) Chari M A and Syamasundar K 2005 Synthesis 708; (j) Reddy S M, Reddy Y V and Venkateswarlu Y 2005 Tetrahedron Lett. 46 7439; (k) Maddani M R and Prabhu K R 2011 Synlett 6 821; (l) Yadav J S, Satyanarayana M, Raghavendra S and Balanarsaiah E 2005 Tetrahedron Lett. 46 8745; (m) Fleet G W J and Witty D R 1990 Tetrahedron: Asymmetry 1 119; (n) Rajput V K, Roy B and Mukhopadhyay B 2006 Tetrahedron Lett. 47 6987; (o) Barrades J S, Errea M I and Accorso N B D 2012 Carbohyd. Res. 355 79; (p) Shing T K M and Leung G Y C 2002 Tetrahedron 58 7545; (q) Sharma G V M, Yadav T A, Choudhury M and Kunwar A C 2012 J. Org. Chem. 77 6834; (r) Christiane M and Carreira E M 2005 J. Am. Chem. Soc. 127 11505
(a) Maiti G and Roy S C 1996 J. Org. Chem. 61 6038; (b) Mandal P K, Dutta P and Roy S C 1997 Tetrahedron Lett. 38 7271; (c) Jana S and Roy S C 2007 Indian J. Chem. 46B 707
(a) Habibi M, Tangestaninejad S, Baltork I, Mirkhani V and Yadollahi B 2001 Tetrahedron Lett. 42 6771; (b) Li J, Wu Y, Fuller N, Markus M and Li W J 2010 Org. Chem. 75 1077; (c) Yuan C, Yang Li, Yue G, Yu T, Zhong W and Liu B 2012 Tetrahedron Lett. 53 6972
Acknowledgements
We thank the Department of Science and Technology (DST), New Delhi for financial support (SR/S-1/OC-12/2011). SM thanks the Council of Scientific and Industrial Research (CSIR), New Delhi for the award of Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MUKHERJEE, S., SENGUPTA, A. & ROY, S.C. Environment friendly chemoselective deprotection of acetonides and cleavage of acetals and ketals in aqueous medium without using any catalyst or organic solvent. J Chem Sci 125, 1493–1496 (2013). https://doi.org/10.1007/s12039-013-0514-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0514-7