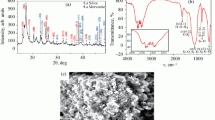
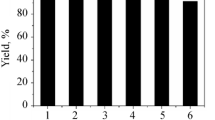
Abstract
The Baylis–Hillman reaction using ionic liquid/hydrotalcite clay catalytic system has been observed to be more reactive in terms of yield and reaction rate than DABCO/acetonitrile system. During the process, the reactants enjoy ionic liquid/hydrotalcite clay catalytic system and gives corresponding Baylis–Hillman reaction products in good yield. The application of our catalytic system has been diversifying for the synthesis of lactone ceramide analogue from (S)-Garner aldehyde-methyl acrylate using Baylis–Hillman reaction. Recycling of ionic liquid/hydrotalcite clay catalytic system has also been demonstrated in this report.

The Baylis–Hillman reaction using ionic liquid/HT-clay catalytic system has been shown to be more reactive in terms of yield and reaction rate than DABCO/acetonitrile system.



Similar content being viewed by others
References
Welton T and Wassersheid P 2008 Ionic liquids in synthesis, 2nd edition (Weinheim: Wiley-VCH) 488
Johnson K E 2007 Electrochem. Soc. Interface 16 38
Wagner M 2004 Chim. Oggi. 22 17
(a) Srivastava V 2010 Cent. Eur. J. Chem. 8 269; (b) Loh T P, Feng L C and Yang H Y 2002 Tetrahedron Lett. 43 8741; (c) Maria M, Toma S, Berkessel A and Koch B 2006 Lett. Org. Chem. 3 437; (d) Guo H M, Cun L F, Gong L Z, Mi A Q and Jiang Y Z 2005 Chem. Commun. 1450; (e) Toma S, Meciarová M and Sebesta R 2009 Eur. J. Org. Chem. 321; (f) Kitazume T, Jiang Z, Kasai K, Mihara Y and Suzuki M 2003 J. Fluorine Chem. 212 205
(a) Prechtl M H G, Scholten J D and Dupont J 2010 Molecules 15 3441; (b) Sowmiah S, Srinivasadesikan V, Tseng M C and Chu Y H 2009 Molecules 14 3780; (c) Karimi B and Zamani A 2012 Org. Biomol. Chem. 10 4531
Hangarge R V, Jarikote D V and Shingare M S 2002 Green Chem. 4 266
(a) Jeong Y and Ryu J S 2010 J. Org. Chem. 75 4183; (b) Rosa J N, Afonso C A M and Santos A G 2001 Tetrahedron 57 4189
Earle M J, McCormac P B and Seddon K R 1999 Green Chem. 1 23
(a) George S M 1995 Chem. Rev. 95 475; (b) Thomas J M and Thomas W J 1996 Principles and practice of heterogeneous catalysis (Weinheim: Wiley-VCH); (c) Hagen J 2006 Industrial catalysis: A practical approach (Weinheim: Wiley-VCH).
(a) Misra C and Perrotta A J 1992 Clays Clay Minerals 40 145; (b) Othman M R, Helwani Z and Fernando W J N 2009 Appl. Organomet. Chem. 23 335; (c) Kannan S 2006 Catal. Surv. Asia 10 117; (d) Debecker D P, Gaigneaux E M and Busca G 2009 Chemistry 15 3920; (e) Srivastava V 2012 Bull. Catal. Soc. India 11 33
(a) Kantam M L, Choudary B M, Reddy C K V, Rao K K and Figueras F 1998 Chem. Commun. 1033; (b) Bulbule V J, Deshpande V H, Velu S, Sudalai A, Sivasanka S and Sate V T 1999 Tetrahedron 55 9325 and references cited therein; (c) Kumbhar P S, Valente J S and Figueras F 1998 Chem. Commun., 1091; (d) Kumbhar P S, Valente J S and Figueras F 1998 Chem. Commun. 535; (e) Ueno S, Yamaguch K, Yoshida K, Ebitani K and Kaneda K 1998 Chem. Commun. 295
(a) Basavaiah D and Raghavaiah G V 2012 Chem. Soc. Rev. 41 68; (b) Singh V and Batra S 2008 Tetrahedron 64 4511; (c) Shi M, Wang F, Zhao M-X and Wei Y 2011 Chemistry of the Morita–Baylis–Hillman reaction RSC Catalysis Series; (d) Basavaiah D, Reddy B S and Badsara S S 2010 Chem. Rev. 110 5447; (e) Basavaiah D, Rao K V and Reddy R J 2007 Chem. Soc. Rev. 36 1581
(a) Aggarwal V K and Meeru A 1999 Chem. Commun. 2311; (b) Kataoka T, Iwama T, Kinoshita H, Surukami S, Iwwamura T and Watanabe S 1999 Synlett 197; (c) Hayase T, Shibata T S, Soai K and Wakatsuki Y 1998 Chem. Commun. 1271; (d) Li G, Wei H-X, Gao J J and Caputo T D 2001 Tetrahedron Lett. 41 1; (e) Chen S H, Hong B C, Su C F and Sarshar S 2005 Tetrahedron Lett. 46 8899; (f) Rosa J N, Afonso A M and Santos A G 2001 Tetrahedron 57 4189; (g) Aggarwal V K, Emme I and Mereu A 2002 Chem. Cummun. 1612
(a) Aravind A, Sanil George G and Kumar S K 2007 Chem. Central J. 1 435; (b) Giacalone F, Gruttadauria M, Marculescu A M, D’Anna F and Noto R 2008 Catal. Commun. 9 1477; (c) Hullio A A and Mastoi G M 2001 Jordan J. Chem. 7 125; (d) Sammelson R E and Kurth M 2001 J. Chem. Rev. 101 137
(a) Srivastava V, Gaubert K, Pucheault M and Vaultier M 2009 Chem. Cat. Chem. 98 94; (b) Khan F A, Dash J, Satapathy R and Upadhyay S K 2004 Tetrahedron Lett. 45 3055; (c) Srivastava V 2013 J. Chem. 2013 1 (Article ID 439673); (d) Srivastava V 2013 J. Chem. 2013 1 (Article ID 954094); (e) Srivastava V 2012 Asymmetric Organocatalysis 1 2; (f) Kim K H, Lee, H S, Kim Y M and Kim J N 2011 Bull. Korean Chem. Soc, 32 1087
Kumar G, Kaur S and Singh V 2011 ARKIVOC 148
Verron J, Joerger J M, Pucheault M and Vaultier M 2007 Tetrahedron Lett. 48 4055
Miyata S 1975 Clays Clay Minerals 369
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
SRIVASTAVA, V. Recyclable hydrotalcite clay catalysed Baylis–Hillman reaction. J Chem Sci 125, 1207–1212 (2013). https://doi.org/10.1007/s12039-013-0472-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0472-0