Abstract
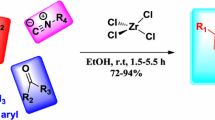
Quaternary ammonium salt mediated highly regioselective ring opening of aziridines with zinc(II) halides to racemic and non-racemic β-halo amines in excellent yield and selectivity is described. The reaction proceeds via an SN2-type pathway and the partial racemization of the starting substrate and the product was effectively controlled by using quaternary ammonium salts to afford the enantioenriched products (er up to 95:5).

Quaternary ammonium salt mediated highly regioselective ring opening of aziridines with zinc(II) halides to racemic and non-racemic β-halo amines in excellent yield and selectivity is described.
Similar content being viewed by others
References
For some reviews of syntheses and reactions of activated and nonactivated aziridines see: (a) Padwa A, Pearson W H, Lian B W and Bergmeier S C 1996 Comprehensive heterocyclic chemistry, II, A R Katritzky, C W Rees and E F V Scriven (eds) New York, Pergamon; Vol. 1A, pp 1–60; (b) Padwa A and Woolhouse A D 1984 Comprehensive heterocyclic chemistry, W Lwowski (ed.) Oxford, Pergamon; Vol. 7, pp 47; (c) Aziridines and epoxides in organic synthesis, A K Yudin (ed) 2006, Weinheim, Wiley-VCH, pp 1–184; (d) Tanner D 1994 Angew. Chem. Int. Ed. Engl. 33 599; (e) Ibuka T 1998 Chem. Soc. Rev. 27 145; (f) Li A-H, Dai L-X and Aggarwal V K 1997 Chem. Rev. 97 2341; (g) Stamm H 1999 J. Prakt. Chem. 341 319; (h) Enders D, Janeck C F and Raabe G 2000 Eur. J. Org. Chem. 3337; (i) McCoull W and Davis F A 2000 Synthesis 1347; (j) D’hooghe M, Kerkaert I, Rottiers M and De Kimpe N 2005 Tetrahedron 60 3637; (k) D’hooghe M and De Kimpe N 2007 Chem. Commun. 1275; (l) Blyumin E V, Gallon H J and Yudin A K 2007 Org. Lett. 9 4677; (m) Singh G S, D’hooghe M and De kimpe N 2007 Chem. Rev. 107 2080; (n) Paixão M W, Nielsen M, Jacobsen C B and Jørgensen K A 2008 Org. Biomol. Chem. 6 3467; (o) Leemans E, Mangelinckx S and De Kimpe N 2009 Synlett. 8 1265; (p) Alcaide B and Almendros P 2009 Progress in heterocyclic chemisty, G W Gribble and J A Joules (eds) Oxford, UK, Elsevier, Vol. 20, pp 74; (q) Minakata S, Murakami Y, Satake M, Hidaka I, Okada Y and Komatsu M 2009 Org. Biomol. Chem. 7 641; (r) Xu Y, Lin L, Kanai M, Matsunaga S and Shibasaki M 2011 J. Am. Chem. Soc. 133 5791; (s) Ghorai M K, Nanaji Y and Yadav A K 2011 Org. Lett. 13 4256
For ring opening of aziridines: (a) Hu X E 2004 Tetrahedron 60 2701 and references cited therein; (b) Minakata S, Okada Y, Oderaotoshi Y and Komatsu M 2005 Org. Lett. 7 3509; (c) Ding C-H, Dai L-X and Hou X-L 2005 Tetrahedron 61 9586; (d) Pineschi M, Bertolini F, Haak R M, Crotti P and Macchia F 2005 Chem. Commun. 1426; (e) Minakata S, Hotta T, Oderaotoshi Y and Komatsu M 2006 J. Org. Chem. 71 7471; (f) Fukuta Y, Mita T, Fukuda N, Kanai M and Shibasaki M 2006 J. Am. Chem. Soc. 128 6312; (g) Crestey F, Witt M, Jaroszewski J W and Franzyk H 2009 J. Org. Chem. 74 5652; (h) Wang Z, Cui Y-T, Xu Z-B and Qu J 2008 J. Org. Chem. 73 2270; (i) Moss T A, Fenwick D R and Dixon D J 2008 J. Am. Chem. Soc. 130 10076; (j) Sureshkumar D, Ganesh V, Vidyarini R S and Chandrasekaran S 2009 J. Org. Chem. 74 7958; (k) D’hooghe M, Vervisch K and De Kimpe N 2007 J. Org. Chem. 72 7329; (l) Banks H D 2010 J. Org. Chem. 75 2510; (m) Bera M and Roy S 2010 J. Org. Chem. 75 4402; (n) Forbeck E M, Evans C D, Gilleran J A, Li P and Joullié M M 2007 J. Am. Chem. Soc. 129 14463; (o) Ochoa-Terán A, Concellón J M and Rivero I A 2009 (ii) ARKIVOC, 288; (p) Concellón J M, Bernad P L and Suárez J R 2005 J. Org. Chem. 70 9411; (q) Couty F, Evano G and Prim D 2005 Tetrahedron Lett. 46 2253; (r) De Rycke N, David O and Couty F 2011 Org. Lett. 13 1836; D’hooghe M, Kenis S, Vervisch K, Lategan C, Smith P J, Chibale K and De Kimpe N 2011 Eur. J. Med. Chem. 46 579; (s) Yadav J S, Satheesh G and Murthy C V S R 2010 Org. Lett. 12 2544; (t) Concellón J M, Rodriguez-Solla H, Amo V and Diaz P 2010 J. Org. Chem. 75 2407; (u) Zeng F and Alper H 2010 Org. Lett. 12 5567; (v) Karikomi M, D’hooghe M, Verniest G and De Kimpe N 2008 Org. Biomol. Chem. 6 1902; Catak S, D’hooghe M, De Kimpe N, Waroquier M and Speybroeck V V 2010 J. Org. Chem. 75 885; D’hooghe M, Rottiers M, Kerkaert I and De Kimpe N 2005 Tetrahedron 61 8746; D’hooghe M, Waterinckx A, Vanlangendonck T and De Kimpe N 2006 Tetrahedron 62 2295; (w) Bhadra S, Adak L, Samanta S, Islam A K M M, Mukherjee M and Ranu B C 2010 J. Org. Chem. 75 8533; (x) Bera M, Pratihar S and Roy S 2011 J. Org. Chem. 76 1475; (y) Brandi A, Cicchi S, Cordero F M 2008 Chem. Rev. 108 3988; (z) Jiang H, Yuan S, Wan W, Yang K, Deng H and Hao J 2010 Eur. J. Org. Chem. 4227
For cycloaddition of aziridines: (a) Concellón J M, Riego E, Suárez J R, García-Granda S and Díaz M R 2004 Org. Lett. 6 4499; (b) Zhu W, Cai G and Ma D 2005 Org. Lett. 7 5545; (c) Guo H, Xu Q and Kwon O 2009 J. Am. Chem. Soc. 131 6318; (d) Pattenden L C, Wybrow R A J, Smith S A and Harrity J P A 2006 Org. Lett. 8 3089; (e) Kang B, Miller A W, Goyal S and Nguyen S T 2009 Chem. Commun. 3928; (f) Wender P A and Strand D 2009 J. Am. Chem. Soc. 131 7528; For cycloaddition of azetidines: (g) Ungureanu I, Klotz P, Schoenfelder A and Mann A 2001 Chem. Commun. 958; (h) Ungureanu I, Klotz P, Schoenfelder A and Mann A 2001 Tetrahedron Lett. 42 6087; (i) Yadav V K and Sriramurthy V 2005 J. Am. Chem. Soc. 127 16366; (j) Baeg J-O, Bensimon C and Alper H 1995 J. Org. Chem. 60 253
For rearrangement: (a) Alcaide B, Almendros P, Aragoncillo C and Salgado N R 1999 J. Org. Chem. 64 9596 and the references cited therein; (b) Vanecko J A and West F G 2005 Org. Lett. 7 2949; (c) Rosser C M, Coote S C, Kirby J P, O’Brien P and Caine D 2004 Org. Lett. 6 4817; (d) Zhao X, Zhang E, Tu Y-Q, Zhang Y-Q, Yuan D-Y, Cao K, Fan C-A and Zhang F-M 2009 Org. Lett. 11 4002; (e) Sugihara Y, Iimura S and Nakayama J 2002 Chem. Commun. 134; (f) Pindinelli E, Pilati T and Troisi L 2007 Eur. J. Org. Chem. 5926
Ghorai M K, Das K, Kumar A and Ghosh K 2005 Tetrahedron Lett. 46 4103; (b) Ghorai M K and Tiwari D P 2010 J. Org. Chem. 75 6173; (c) Ghorai M K, Das K, Kumar A and Das A 2006 Tetrahedron Lett. 47 5393; (d) Ghorai M K, Ghosh K and Das K 2006 Tetrahedron Lett. 47 5399; (e) Ghorai M K and Ghosh K 2007 Tetrahedron Lett. 48 3191; (f) Ghorai M K, Das K and Kumar A 2007 Tetrahedron Lett. 48 4373; (g) Ghorai M K, Das K and Kumar A 2009 Tetrahedron Lett. 50 1105; (h) Ghorai M K, Kumar A and Das K 2007 Org. Lett. 9 5441; (i) Ghorai M K, Das K, Shukla D 2007 J. Org. Chem. 72 5859; (j) Ghorai M K, Shukla D and Das K 2009 J. Org. Chem. 74 7013 and references cited therein
Ghorai M K, Kumar A and Tiwari D P 2010 J. Org. Chem. 75 137
Narender M, Surendra K, Krishnaveni N S, Reddy M S and Rao K R 2004 Tetrahedron Lett. 45 7995; (b) Das B, Reddy V S and Thirupathi P 2006 J. Mol. Catal. A: Chem. 255 28; (c) Gnecco D, Orea F L, Galindo A, Enríquez R G, Toscano R A and Reynolds W F 2000 Molecules 5 998; (d) Righi G, Franchini T and Bonini C 1998 Tetrahedron Lett. 39 2385; (e) Righi G, Potini C and Bovicelli P 2002 Tetrahedron Lett. 43 5867; (f) Sabitha G, Babu R S, Rajkumar M, Reddy C S and Yadav J S 2001 Tetrahedron Lett. 42 3955; (g) Yadav J S, Reddy B V S and Kumar G M 2001 Synlett 1417; (h) Ding C-H, Dai L-X and Hou X L 2004 Synlett 2218; (i) Das B, Krishnaiah M and Venkateswarlu K 2007 Chem. Lett. 36 82; (j) Kumar M, Pandey S K, Gandhi S and Singh V K 2009 Tetrahedron Lett. 50 363
Concellón J M, Rodríguez-Solla H, Bernad P L and Simal C 2009 J. Org. Chem. 74 2452; (b) D’hooghe M, Vervisch K, Nieuwenhove A V, De Kimpe N 2007 Tetrahedron Lett. 48 1771; (c) D’hooghe M, Aelterman W, De Kimpe N 2009 Org. Biomol. Chem. 7 135 and references cited therein
For some recent examples of haloamination see (a) Spassova M K, Bornmann W G, Ragupathi G, Sukenick G, Livingston P O and Danishefsky S J 2005 J. Org. Chem. 70 3383; (b) De Castro M and Marzabadi C H 2004 Tetrahedron Lett. 45 6501; (c) Yeung Y Y, Gau X and Corey E J 2006 J. Am. Chem. Soc. 128 9644; (d) Raghavan S, Mustafa S and Sridhar B 2009 J. Org. Chem. 74 4499; (e) Rawal G K, Kumar A, Tawar U and Vankar Y D 2007 Org. Lett. 9 5171
For a recent review see (a) Li G, Kotti S R S S and Timmons C 2007 Eur. J. Org. Chem. 2745 and references therein; (b) Han J-L, Zhi S-J, Wang L-Y, Pan Y and Li G 2007 Eur. J. Org. Chem. 1332; (c) Wang Y-N, Ni B, Headley A D and Li G 2007 Adv. Synth. Catal. 349 319; (d) Shaikh T M, Karabal P U, Suryavanshi G and Sudalai A 2009 Tetrahedron Lett. 50 2815
Kemp J E G 1991 Comprehensive organic synthesis, B M Trost and I Fleming (eds) Oxford, Pergamon; Vol. 3, pp 471–513; (b) Owens J M, Yeung B K S, Hill D C and Petillo P A 2001 J. Org. Chem. 66 1484; (c) Griffith D A and Danishefsky S J 1991 J. Am. Chem. Soc. 113 5863
Tang S-S, Simpson D E and Kagan H M 1984 J. Biol. Chem. 259 975; (b) Medda R, Padiglia A, Pedersen J Z, Agraò A F, Rotilio G and Floris G 1997 Biochemistry 36 2595
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
GHORAI, M.K., TIWARI, D.P., KUMAR, A. et al. S N 2-type ring opening of substituted-N-tosylaziridines with zinc (II) halides: Control of racemization by quaternary ammonium salt. J Chem Sci 123, 951–961 (2011). https://doi.org/10.1007/s12039-011-0178-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-011-0178-0