Abstract
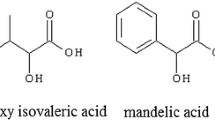
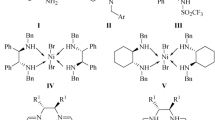
Efficient use of the readily accessible chiral C 2-symmetric acyclic diamines (1–2) as well as macrocyclic amines (3–5) containing trans-1,2-diaminocyclohexyl moiety as chiral solvating agents (CSA) for the determination of enantiomeric excess of representative carboxylic acids (6–7) and an amino acid derivative (8) is illustrated. The enantiomeric composition of different carboxylic acids estimated here by the 1H NMR method, based on the integration of the corresponding methine proton signals are in good correlation with that determined using HPLC method. The data are in accordance with the formation of multimolecular diastereomeric complexes in solution, which render good splitting of NMR signals for the enantiomers of representative carboxylic acids as well as for N-Ts-phenylglycine (up to ΔΔδ = 0.295 ppm, 118 Hz).
Similar content being viewed by others
References
Lin J, Hu Q-S, Xu M-H and Pu L 2002 J. Am. Chem. Soc. 124 2088
Pu L 2004 Chem. Rev. 104 1687
Lin J, Rajaram A R and Pu L 2004 Tetrahedron 60 11277
Valentine Jr D, Johnson K K, Priester W, Sun R C, Toth K and Saucy G 1980 J. Org. Chem. 45 3698
Seco J M, Quiñoá E and Riguera R 2004 Chem. Rev. 104 17
Wenzel T J and Wilcox J D 2003 Chirality 15 256
Parker D 1991 Chem. Rev. 91 1441
Uccello-Barretta G, Balzano F, Sicoli G, Scarselli A and Salvadori P 2005 Eur. J. Org. Chem. 5349
Uccello-Barretta G, Balzano F, Martinelli J, Berni M G, Villani C and Gasparrini F 2005 Tetrahedron: Asymmetry 16 3746
Ema T, Tanida D and Sakai T 2007 J. Am. Chem. Soc. 129 10591
For recent examples of in situ covalent CSRs, which do not require additional purification steps, see: (a) Pérez-Fuertes Y, Kelly A M, Johnson A L, Arimori S, Bull S D and James T D 2006 Org. Lett. 8 609
(b) Chin J, Kim D C, Kim H-J, Panosyan F B and Kim K M 2004 Org. Lett. 6 2591
For example, see: Fraser R R 1983 In Asymmetric synthesis (ed.) J D Morrison (New York: Academic Press) vol. 1
Wenzel T J and Thurston J E 2000 J. Org. Chem. 65 1243
Wenzel T J, Thurston J E, Sek D C and Joly J-P 2001 Tetrahedron: Asymmetry 12 1125
Machida Y, Kagawa M and Nishi H 2003 J. Pharm. Biomed. Anal. 30 1929
Lovely A E and Wenzel T J 2006 J. Org. Chem. 71 9178
Wenzel T J, Amonoo E P, Shariff S S and Aniagyei S E 2003 Tetrahedron: Asymmetry 14 3099
Simonato J-P, Chappellet S, Pecaut J, Baret P and Marchon J-C 2001 New. J. Chem. 25 714
Claeys-Bruno M, Toronto D, Pecaut J, Bardet M, and Marchon J-C 2001 J. Am. Chem. Soc. 123 11067
Schwenninger R, Schlogl J, Maynollo J, Gruber K, Ochsenbein P, Burgi H-B, Konrat R and Krautler B 2001 Chem. Eur. J. 7 2676
Ema T, Ouchi N, Doi T, Korenaga T and Sakai T 2005 Org. Lett. 7 3985
Webb T H and Wilcox C S 1993 Chem. Soc. Rev. 22 383
Zhang X X, Bradshaw J S and Izatt R M 1997 Chem. Rev. 97 3313
Chen G-M, Brown H C and Ramachandran P V 1999 J. Org. Chem. 64 721
Tejeda A, Oliva A I, Simon L, Grande M, Caballero M C and Moran J R 2000 Tetrahedron Lett. 41 4563
Alfonso I, Rebolledo F and Gotor V 2000 Chem. Eur. J. 6 3331
Tsubaki K, Nuruzzaman M, Kusumoto T, Hayashi N, Bin-Gui W and Fuji K 2001 Org. Lett. 3 4071
Lin J, Hu Q-S, Xu M-H and Pu L 2002 J. Am. Chem. Soc. 124 2088
Kim B M, So S M and Choi H J 2002 Org. Lett. 4 949
Ito K, Noike M, Kita A and Ohda Y 2002 J. Org. Chem. 67 7519
Du C-P, You J-S, Yu X-Q, Liu C L, Lan J-B and Xie R-G 2003 Tetrahedron: Asymmetry 14 3651
Yang X, Wu X, Fang M, Yuan Q and Fu E 2004 Tetrahedron: Asymmetry 15 2491
Ema T, Tanida D and Sakai T 2006 Org. Lett. 8 3773
Pirkle W H and Hoekstra M S 1976 J. Am. Chem. Soc. 98 1832
Pirkle W H and Sikkenga D L 1977 J. Org. Chem. 42 1370
Deshmukh M, Dunach E, Juge S and Kagan H B 1984 Tetrahedron Lett. 25 3467
Toda F, Mori K, Okada J, Node M, Itoh A, Oomine K and Fuji K 1988 Chem. Lett. 131
Toda F, Mori K and Sato A 1988 Bull. Chem. Soc. Jpn. 61 4167
Toda F, Toyotaka R and Fukuda H 1990 Tetrahedron: Asymmetry 1 303
Tanaka K, Ootani M and Toda F 1992 Tetrahedron: Asymmetry 3 709
Bilz A, Stork T and Helmchen G 1997 Tetrahedron: Asymmetry 8 3999
Lacour L, Vial L and Herse C 2002 Org. Lett. 4 1351
Pakulski Z, Demchuk O M, Kwiatosz R, Osinski P W, Swierczynska W and Pietrusiewicz K M 2003 Tetrahedron: Asymmetry 14 1459
Hebbe V, Londez A, Goujon-Ginglinger C, Meyer F, Uziel J, Juge S and Lacour J 2003 Tetrahedron Lett. 44 2467
Koscho M E and Pirkle W H 2005 Tetrahedron: Asymmetry 16 3345
Yang D, Li X, Fan Y-F and Zhang D-W 2005 J. Am. Chem. Soc. 127 7996
Palomino-Schatzlein M, Virgili A, Gil S and Jaime C 2006 J. Org. Chem. 71 8114
Perez-Trujillo M and Virgili A 2006 Tetrahedron: Asymmetry 17 2842
Lin J, Zhang H and Pu L 2002 Org. Lett. 4 3297
Cuevas F, Ballester P and Pericas M A 2005 Org. Lett. 7 5485
Yang D, Li X, Fan Y and Zhang D 2005 J. Am. Chem. Soc. 127 7996
Gonzalez-Alvarez A, Alfonso I and Gotor V 2006 Tetrahedron Lett. 47 6397
Ma F, Ai L, Shen X and Zhang C 2007 Org. Lett. 9 125
Ema T, Tanida D and Sakai T 2007 J. Am. Chem. Soc. 129 10591
Luo Z, Zhong C, Wu X and Fu E 2008 Terahedron Lett. 49 3385
Pena C, Gonzalez-Sabin J, Alfonso I, Rebolledo F and Gotor V 2008 Tetrahedron 64 7709
Tanaka K, Fukuda N and Fujiwara T 2007 Tetrahedron: Asymmetry 18 2657
Tanaka K and Fukuda N 2009 Tetrahedron: Asymmetry 20 111
Zhang X-X, Bradshaw J S and Izatt R M 1997 Chem. Rev. 97 3313
Padmaja M and Periasamy M 2004 Tetrahedron: Asymmetry 15 2437
Dalai M and Periasamy M 2009 Tetrahedron: Asymmetry 20 1247
Gawronski J, Kolbon H, Kwit M and Katrusiak A 2000 J. Org. Chem. 65 5768
Pine S H and Sanchez B L 1971 J. Org. Chem. 36 829
Prasad A S B, Kanth J V B and Periasamy M 1992 Tetrahedron 48 4623
For the synthesis of a similar type of C 2-symmetric tetraazamacrocycle containing 1,2-diaminocyclohexane moiety with aliphatic spacers see Alfonso I, Astorga C, Rebolledo F and Gotor V 1999 Tetrahedron: Asymmetry 10 2515
González-álvarez A, Alfonso I, Díaz P, García-España E and Gotor V 2006 Chem. Commun. 1227
Ramanathan C R and Periasamy M 1998 Tetrahedron: Asymmetry 9 2651
Larrow J F, Jacobsen E N, Gao Y, Hong Y, Nie X and Zepp C M 1994. J. Org. Chem. 59 1939
Matsumura Y, Nishimura M, Hiu H, Watanabe M and Kise N 1996 J. Org. Chem. 61 2809
Ebbers E J, Ariaans G J A, Bruggink A and Zwanenburg B 1999 Tetrahedron: Asymmetry 10 3701
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s12039-011-0144-x
Rights and permissions
About this article
Cite this article
Periasamy, M., Dalai, M. & Padmaja, M. Chiral trans-1,2-diaminocyclohexane derivatives as chiral solvating agents for carboxylic acids. J Chem Sci 122, 561–569 (2010). https://doi.org/10.1007/s12039-010-0090-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-010-0090-z