Abstract
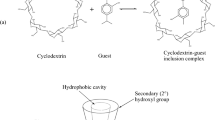
Loperamide (LPR) is a synthetic, poorly water soluble, peripherally acting opiate agonist drug used for the treatment of diarrhea. Major challenges in formulating this drug for clinical applications include solubility enhancement and improved stability in biological systems. Cyclodextrins (CDs) are chiral, truncated cone shaped; cyclic oligosaccharides that can encapsulate a variety of poorly water soluble drug molecules into inclusion complexes, thereby increasing their stability and solubility. 1H NMR spectroscopic studies showed the inclusion complexation between β-CD and LPR, based on the upfield shift changes in the β-CD cavity protons (H-3′ and H-5′) and downfield shift changes in the guest (LPR) protons. 2D COSY spectral data was used for assignment of β-CD as well as LPR protons and 2D ROESY spectral data to know the inclusion of LPR inside the β-CD cavity. The 1: 1 stoichiometry and overall association constant (K a) were determined by using Scott’s plot method to be 68·805 M−1. 2D ROESY spectral data suggest that the inclusion of aromatic rings of LPR in β-CD cavity can be from narrower as well as the wider rim side and the six possible 1: 1 LPR: β-CD inclusion complexes have been proposed. Thus, we anticipate that complexation of LPR with β-CD would increase its solubility and stability in biological system.
Similar content being viewed by others
References
Heel R C, Brogden R N, Speight T M and Avery G S 1979 Drugs 15 33
Dodziuk H 2006 Cyclodextrins and their complexes. chemistry, analytical methods, applications (London: Wiley-VCH)
Schneider H-J, Hacket F, Rudiger V and Ikeda H 1998 Chem. Rev. 98 1755
Connors K A 1987 Binding constants: the measurement of molecular complex stability (New York: Wiley)
Fielding L 2000 Tetrahedron 56 6151
Ali S M and Upadhyay, S K 2008 Magn. Reson. Chem. 46 676
Higuchi T and Connors K A 1965 Adv. Anal. Chem. Instr. 4 117
Scott R L 1956 Recl. Trav. Chim Pays-Bas 75 787
Benesi H A and Hildebrand J H 1949 J. Am. Chem. Soc. 71 2703
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Upadhyay, S.K., Ali, S.M. Solution structure of loperamide and β-cyclodextrin inclusion complexes using NMR spectroscopy. J Chem Sci 121, 521–527 (2009). https://doi.org/10.1007/s12039-009-0063-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-009-0063-2