Abstract
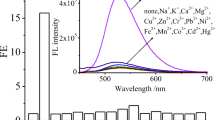
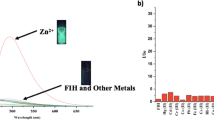
Fluorescence behaviour of 4-benzoylamido-N-methylphthalimide (1), designed and developed for selective detection of fluoride ions, is reported. 1 displays F−-induced colour change that allows its detection with the naked eye. The F− specificity of the sensor system is evident from the fact that unlike F−, other halides do not affect the absorption characteristics of 1. Apart from the colorimetric response, the fluorescence output of 1 is also modulated by F− in a manner that permits ratiometric fluorescence signalling of F− as well. It is found that the system can detect F− in the concentration range of 10–60 μM. The results of the experiments and theoretical calculations unambiguously suggest that the changes of the electronic absorption and fluorescence behaviour of 1, which have been exploited for signalling purpose, are due to F−-induced deprotonation of the 4-amido moiety of the sensor system.
Similar content being viewed by others
References
Beer P D and Cadman J 2000 Coord. Chem. Rev. 205 131
Schmidtchen F P and Berger M 1997 Chem. Rev. 97 1609
Gale P A 2001 Coord. Chem. Rev. 213 79
Davis A P and Wareham R S 1999 Angew. Chem., Int. Ed. 38 2978
Martinez-Manez R and Sancenon F 2003 Chem. Rev. 103 4419
Sukasai C and Tuntulani T 2003 Chem. Soc. Rev. 32 192
Lee D H, Lee H Y and Hong J-I 2002 Tetrahedron Lett. 43 7273
Gale P A 2000 Coord. Chem. Rev. 199 181
Amendola V, Gomez D E, Fabrizi L and Licchelli M 2006 Acc. Chem. Res. 39 343
Pordi L, Bolletta F, Montalti M and Zaccheroni N 2000 Coord. Chem. Rev. 199 181
Valeur B and Leray I 2000 Coord. Chem. Rev. 205 3
de Silva A P, Fox D B, Huxley A J M and Moody T S 2000 Coord. Chem. Rev. 205 41
Rurack K 2001 Spectrochim. Acta A57 2161
Keefe M H, Benkstein K D and Hupp J T 2000 Coord. Chem. Rev. 205 201
Robertson A and Shinkai S 2000 Coord. Chem. Rev. 205 157
de Silva A P and Tecilla P (eds) 2005 Special issue on fluorescent sensors. J. Mater. Chem. 15 265
Ramachandram B and Samanta A 1997 Chem. Commun 1037
Sankaran N B, Banthia S, Das A and Samanta A 2002 New J. Chem. 26 1529
Banthia S and Samanta A. 2002 J. Phys. Chem. B106 5572
Banthia S, Sarkar M and Samanta A 2005 Res. Chem. Intermed. 31 25
Banthia S and Samanta A 2006 J. Phys. Chem. B110 6437
Kirk K L 1991 Biochemistry of the halogens and inorganic halides (New York: Plenum) p. 58
Kleerekoper M 1998 Endocrinol. Metab. Clin. North Am. 27 441
Wiseman A 1970 Handbook of experimental pharmacology (Berlin: Springer-Verleg) vol. 20, part 2, p. 48
Ghosh T, Maiya B G and Wong M W 2004 J. Phys. Chem. A108 11249
Gunnlaugsson T, Kruger P E, Lee T C, Prakesh R, Pfeffer F M and Hussey G M 2003 Tetrahodron Lett. 44 6575
Chen Q and Chen C 2004 Tetrahedron Lett. 45 6493
Coskun A and Akkaya E 2004 Tetrahedron Lett. 45 4947
Gale P, Sessler J L and Vladimir K 1998 Chem. Commun. 1
Biocchi M, Boca L D, Gomez D E, Fabbrizzi L, Liccheli M and Monazani E 2004 J. Am. Chem. Soc. 126 16507
Jose D A, Kumar D K, Ganguly B and Das A 2004 Org. Lett. 6 3445
Jose D A, Kumar D K, Ganguly B and Das A 2005 Tetrahedron Lett. 46 5343
Ding J and Day M 2006 Macromolecules 39 6054
Arimori S, Davidson M G, Fyles T M, Hibberet T G, James T D and Kociok-Köhn 2004 Chem. Commun. 1640
Ren J, Wang Q, Qu D, Zhao X and Tian H 2004 Chem. Lett. 33 974
Xu G and Tarr M A 2004 Chem. Commun. 1050
Kovalchuk A, Bricks J L, Reck G, Rurack K, Schulz B, Szumna A and WeiBhoff H 2004 Chem. Commun. 1946
Liu B and Tian H 2005 J. Mater. Chem. 15 2681
Soujanya T, Fessenden R W and Samanta A 1996 J. Phys. Chem. 100 3507
Frish M J et al 2003 Gaussian 03 Revision B-05 Gaussian Inc. Pittsburgh, PA
This value is lower than that obtained using AM1 calculation. See for example, ref. [39]
Brooks S J, Evans L S, Gale P A and Hursthouse M B 2004 Chem. Commun. 734
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sarkar, M., Yellampalli, R., Bhattacharya, B. et al. Ratiometric fluorescence signalling of fluoride ions by an amidophthalimide derivative. J Chem Sci 119, 91–97 (2007). https://doi.org/10.1007/s12039-007-0015-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-007-0015-7