Abstract
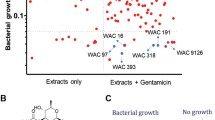
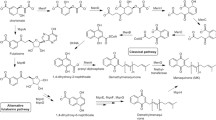
The emergence of resistance to frontline antibiotics has called for novel strategies to combat serious pathogenic infections. Methicillin-resistant Staphylococcus aureus [MRSA] is one such pathogen. As opposed to traditional antibiotics, bacteriostatic anti-virulent agents disarm MRSA, without exerting pressure, that cause resistance. Herein, we employed a thermophilic Thermotoga maritima tryptophan synthase (TmTrpB1) enzyme followed by an isonitrile synthase and Fe(II)-α-ketoglutarate-dependent oxygenase, in sequence as biocatalysts to produce antivirulent indole vinyl isonitriles. We report on conversion of simple derivatives of indoles to their C3-vinyl isonitriles, as the enzymes employed here demonstrated broader substrate tolerance. In toto, eight distinct L-Tryptophan derived α-amino acids (7) were converted to their bioactive vinyl isonitriles (3) by action of an isonitrile synthase (WelI1) and an Fe(II)-α-ketoglutarate-dependent oxygenase (WelI3) yielding structural variants possessing antivirulence against MRSA. These indole vinyl isonitriles at 10 μg/mL are effective as antivirulent compounds against MRSA, as evidenced through analysis of rabbit blood hemolysis assay. Based on a homology modelling exercise, of enzyme-substrate complexes, we deduced potential three dimensional alignments of active sites and glean mechanistic insights into the substrate tolerance of the Fe(II)-α-ketoglutarate-dependent oxygenase.
Graphic abstract









Similar content being viewed by others
References
Alkhalaf LM and Ryan KS 2015 Biosynthetic manipulation of tryptophan in bacteria: pathways and mechanisms. Chem. Biol. 22 317–328
Bhat V, Dave A, MacKay JA, and Rawal VH 2014 The chemistry of hapalindoles, fischerindoles, ambiguines, and welwitindolinones; in The alkaloids: chemistry and biology (ed) H-J Knölker (Academic Press) pp 65–160
Bornemann V, Patterson GML and Moore RE 1988 Isonitrile biosynthesis in the cyanophyte Hapalosiphon fontinalis. J. Am. Chem. Soc. 110 2339–2340
Brady SF, Bauer JD, Clarke-Pearson MF and Daniels R 2007 Natural products from isnA-Containing biosynthetic gene clusters recovered from the genomes of cultured and uncultured bacteria. J. Am. Chem. Soc. 129 12102–12103
Brady SF and Clardy J 2005 Cloning and heterologous expression of isocyanide biosynthetic genes from environmental DNA. Angew. Chemie Int. Ed. 44 7063–7065
Bunn MB 2016 Unraveling genetically encoded pathways leading to bioactive metabolites in group V cyanobacteia. Ph. D. Thesis, Case Western Reserve University, Ohio
Chang W-C, Liu X, et al. 2017 In vitro stepwise reconstitution of amino acid derived vinyl isocyanide biosynthesis: detection of an elusive intermediate. Org. Lett. 19 1208–1211
Clarke-Pearson MF and Brady SF 2008 Paerucumarin, a new metabolite produced by the pvc gene cluster from Pseudomonas aeruginosa. J. Bacteriol. 190 6927
Drake EJ and Gulick AM 2008 Three-dimensional structures of Pseudomonas aeruginosa PvcA and PvcB, two proteins involved in the synthesis of 2-Isocyano-6,7-dihydroxycoumarin. J. Mol. Biol. 384 193–205
Gordon RJ and Lowy FD 2008 Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 46 S350–S359
Hettwer S and Sterner R 2002 A novel tryptophan synthase β-Subunit from the hyperthermophile Thermotoga maritima. J. Biol. Chem. 277 8194–8201
Hillwig ML and Liu X 2014 A new family of iron-dependent halogenases acts on freestanding substrates. Nat. Chem. Biol. 10 921–923
Hillwig ML, Zhu Q, Ittiamornkul K and Liu X 2016 Discovery of a promiscuous non-heme iron halogenase in ambiguine alkaloid biogenesis: implication for an evolvable enzyme family for late-stage halogenation of aliphatic carbons in small molecules. Angew. Chemie Int. Ed. 55 5780–5784
Hillwig ML, Zhu Q and Liu X 2014 Biosynthesis of ambiguine indole alkaloids in cyanobacterium fischerella ambigua. ACS Chem. Biol. 9 372–377
Hoppe I and Schöllkopf U 1984 Synthesis and biological activities of the antibiotic B 371 and its analogs. Liebigs Ann. der Chemie 1984 600–607
Ittiamornkul K, Zhu Q, Gkotsi DS, Smith DRM, Hillwig ML, Nightingale N, Goss RJM and Liu X 2015 Promiscuous indolyl vinyl isonitrile synthases in the biogenesis and diversification of hapalindole-type alkaloids. Chem. Sci. 6 6836–6840
Keasling JD 2010 Manufacturing molecules through metabolic engineering. Science 330 1355
Keasling JD, Mendoza A and Baran PS 2012 A constructive debate. Nature 492 188–189
Kuo D, et al. 2015 Novel quorum-quenching agents promote methicillin-resistant Staphylococcus aureus (MRSA) wound healing and sensitize MRSA to β-lactam antibiotics. Antimicrob. Agents Chemother. 59 1512
Kwon SJ, Mora-Pale M, Lee M-Y and Dordick JS 2012 Expanding nature’s small molecule diversity via in vitro biosynthetic pathway engineering. Curr. Opin. Chem. Biol. 16 186–195
Li S, et al. 2015 Hapalindole/Ambiguine biogenesis is mediated by a cope rearrangement, C-C bond-forming cascade. J. Am. Chem. Soc. 137 15366–15369
Liu X, Hillwig ML, Koharudin LMI and Gronenborn AM 2016 Unified biogenesis of ambiguine, fischerindole, hapalindole and welwitindolinone: identification of a monogeranylated indolenine as a cryptic common biosynthetic intermediate by an unusual magnesium-dependent aromatic prenyltransferase. Chem. Commun. 52 1737–1740
Lu Z, Yang M, Chen P, Xiong X and Li A 2014 Total synthesis of hapalindole-type natural products. Angew. Chemie Int. Ed. 53 13840–13844
Maimone TJ, Ishihara Y and Baran PS 2015 Scalable total syntheses of (-)-Hapalindole U and (+)-Ambiguine H. Tetrahedron 71 3652–3665
Micallef ML, Sharma D, Bunn BM, Gerwick L, Viswanathan R and Moffitt MC 2014 Comparative analysis of hapalindole, ambiguine and welwitindolinone gene clusters and reconstitution of indole-isonitrile biosynthesis from cyanobacteria. BMC Microbiol. 14 213
Moore RE, et al. 1987 Hapalindoles, antibacterial and antimycotic alkaloids from the cyanophyte Hapalosiphon fontinalis. J. Org. Chem. 52 1036–1043
Moore RE, Cheuk C and Patterson GML 1984 Hapalindoles: new alkaloids from the blue-green alga Hapalosiphon fontinalis. J. Am. Chem. Soc. 106 6456–6457
Ongley SE, Bian X, Zhang Y, Chau R, Gerwick WH, Müller R and Neilan BA 2013 High-Titer heterologous production in E. coli of lyngbyatoxin, a protein kinase c activator from an uncultured marine cyanobacterium. ACS Chem. Biol. 8 1888–1893
Otto M 2010 Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus Annu. Rev. Microbiol. 64 143–162
Park A, Moore RE and Patterson GML 1992 Fischerindole L, a new isonitrile from the terrestrial blue-green alga Fischerella muscicola. Tetrahedron Lett. 33 3257–3260
Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamas T, Pohjakallio A and Baran PS 2008 Enantiospecific total synthesis of the hapalindoles, fischerindoles, and welwitindolinones via a redox economic approach. J. Am. Chem. Soc. 130 17938–17954
Savile CK, et al. 2010 Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 329 305
Scheuer PJ 1992 Isocyanides and cyanides as natural products. Acc. Chem. Res. 25 433–439
Simpson JS and Garson MJ 2004 Biosynthetic pathways to isocyanides and isothiocyanates; precursor incorporation studies on terpene metabolites in the tropical marine sponges Amphimedon terpenensis and Axinyssa n. sp. Org. Biomol. Chem. 2 939–948
Spallarossa M, Wang Q, Riva R and Zhu J 2016 Synthesis of vinyl isocyanides and development of a convertible isonitrile. Org. Lett. 18 1622–1625
Tommasi R, Brown DG, Walkup GK, Manchester JI and Miller AA 2015 ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 14 529–542
Wainman YA, Neves AA, Stairs S, Stöckmann H, Ireland-Zecchini H, Brindle KM and Leeper FJ 2013 Dual-sugar imaging using isonitrile and azido-based click chemistries. Org. Biomol. Chem. 11 7297–7300
Walton K and Berry J 2016 Indole alkaloids of the stigonematales (Cyanophyta): chemical diversity, biosynthesis and biological activity. Mar. Drugs 14 73
Wilson RM, Stockdill JL, Wu X, Li X, Vadola PA, Park PK, Wang P and Danishefsky SJ 2012 A fascinating journey into history: exploration of the world of isonitriles en route to complex amides. Angew. Chem. Int. Ed. Engl. 51 2834–2848
Yu G, Kuo D, Shoham M and Viswanathan R 2014 Combinatorial synthesis and in vitro evaluation of a biaryl hydroxyketone library as antivirulence agents against MRSA. ACS Comb. Sci. 16 85–91
Zhang Z, Ren J, Stammers DK, Baldwin JE, Harlos K and Schofield CJ 2000 Structural origins of the selectivity of the trifunctional oxygenase clavaminic acid synthase. Nat. Struct. Biol. 7 127–133
Zhu J, Lippa GM, Gulick AM and Tipton PA 2015 Examining reaction specificity in PvcB, a source of diversity in isonitrile-containing natural products. Biochemistry 54 2659–2669
Acknowledgments
The authors thank Prof. C Dale Poulter for generous gift of TmTrpB1 plasmid. The authors thank the Indiana University Mass Spectrometry facility for assistance with LC-MS and MS-MS data acquisition. BMB thanks the Department of Education (DOE) grant support through a GAANN fellowship (2012–2013). RV is grateful for funding from the Case Western Reserve University’s Start-Up award and support from the Dean’s Office for Inclusion Diversity Faculty Excellence achievement award. RV acknowledges the support from IISER Tirupati towards this publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by BJ RAO .
Corresponding editor: BJ RAO
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bunn, B.M., Xu, M., Webb, C.M. et al. Biocatalysts from cyanobacterial hapalindole pathway afford antivirulent isonitriles against MRSA. J Biosci 46, 37 (2021). https://doi.org/10.1007/s12038-021-00156-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-021-00156-4