Abstract
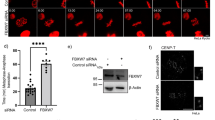
As a tumor suppressor, p53 preserves genomic integrity in eukaryotes. However, limited evidence is available for the p53 shuttling between the cytoplasm and nucleus. Previous studies have shown that β-actin polymerization negatively regulates p53 nuclear import through its interaction with p53. In this study, we found that DNA damage induces both β-actin and p53 accumulation in the nucleus. β-actin knockdown impaired the nuclear transport of p53. Additionally, β-actin could interact with p53 which was enhanced in response to genotoxic stress. Furthermore, N terminal deletion mutants of p53 shows reduced levels of association with β-actin. We further identified Ser15, Thr18 and Ser20 of p53 are critical to the β-actin: p53 interaction, which upon mutation into alanine abrogates the binding. Taken together, this study reveals that β-actin regulates the nuclear import of p53 through protein–protein interaction.




Similar content being viewed by others
Abbreviations
- γH2AX:
-
phosphorylated histone H2AX
- MDM2:
-
the Murine Double Minute 2 oncogene
- Mot2:
-
mortalin 2
- ETO:
-
etoposide
- FBS:
-
fetal bovine serum
- DAPI:
-
4′,6′-diamidino-2 phenylindole
References
Alarcon-Vargas D and Ronai Z 2002 p53-Mdm2–the affair that never ends. Carcinogenesis 23 541–547
Beckerman R and Prives C 2010 Transcriptional regulation by p53. Cold Spring Harb. Perspect. Biol. 2 a000935
Bullock AN, Henckel J, DeDecker BS, Johnson CM, Nikolova PV, Proctor MR, Lane DP and Fersht AR 1997 Thermodynamic stability of wild-type and mutant p53 core domain. Proc. Natl. Acad. Sci. USA 94 14338–14342
Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV and Fojo T 2000 p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat. Cell Biol. 2 709–717
Gottlieb TM and Oren M 1996 p53 in growth control and neoplasia. Biochim. Biophys. Acta 1287 77–102
Guettler S, Vartiainen MK, Miralles F, Larijani B and Treisman R 2008 RPEL motifs link the serum response factor cofactor MAL but not myocardin to Rho signaling via actin binding. Mol. Cell Biol. 28 732–742
Jiang M, Axe T, Holgate R, Rubbi CP, Okorokov AL, Mee T and Milner J 2001 p53 binds the nuclear matrix in normal cells: binding involves the proline-rich domain of p53 and increases following genotoxic stress. Oncogene 20 5449–5458
Joerger AC and Fersht AR 2010 The tumor suppressor p53: from structures to drug discovery. Cold Spring Harb. Perspect. Biol. 2 a000919
Kar S, Sakaguchi K, Shimohigashi Y, Samaddar S, Banerjee R, Basu G, Swaminathan V, Kundu TK and Roy S 2002 Effect of phosphorylation on the structure and fold of transactivation domain of p53. J. Biol. Chem. 277 15579–15585
Liu WL, Midgley C, Stephen C, Saville M and Lane DP 2001 Biological significance of a small highly conserved region in the N terminus of the p53 tumour suppressor protein. J. Mol. Biol. 313 711–731
Metcalfe S, Weeds A, Okorokov AL, Milner J, Cockman M and Pope B 1999 Wild-type p53 protein shows calcium-dependent binding to F-actin. Oncogene 18 2351–2355
O’Brate A and Giannakakou P 2003 The importance of p53 location: nuclear or cytoplasmic zip code? Drug Resist. Updat. 6 313–322
Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC and Hainaut P 2002 The IARC TP53 database: new online mutation analysis and recommendations to users. Hum. Mutat. 19 607–614
Polley S, Guha S, Roy NS, Kar S, Sakaguchi K, Chuman Y, Swaminathan V, Kundu T and Roy S 2008 Differential recognition of phosphorylated transactivation domains of p53 by different p300 domains. J. Mol. Biol. 376 8–12
Qi W, Chen H, Xiao T, Wang R, Li T, Han L and Zeng X 2016 Acetyltransferase p300 collaborates with chromodomain helicase DNA-binding protein 4 (CHD4) to facilitate DNA double-strand break repair. Mutagenesis 31 193–203
Saha T, Guha D, Manna A, Panda AK, Bhat J, Chatterjee S and Sa G 2016 G-actin guides p53 nuclear transport: potential contribution of monomeric actin in altered localization of mutant p53. Sci. Rep. 6 32626
Saha T, Kar RK and Sa G 2015 Structural and sequential context of p53: A review of experimental and theoretical evidence. Prog. Biophys. Mol. Biol. 117 250–263
Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW and Appella E 1998 DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 12 2831–2841
Tian X, Qi W, Chen H, Zeng X, Han L and Mi D 2016 β-Actin regulates interleukin 6-induced p21 transcription by interacting with the Rpb5 and Rpb7 subunits of RNA polymerase II. Anim. Cell. Syst. 20 282–288
Wadhwa R, Takano S, Robert M, Yoshida A, Nomura H, Reddel RR, Mitsui Y and Kaul SC 1998 Inactivation of tumor suppressor p53 by mot-2, a hsp70 family member. J. Biol. Chem. 273 29586–29591
Waldman T, Kinzler KW and Vogelstein B 1995 p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 55 5187–5190
Wang L, Wang M, Wang S, Qi T, Guo L, Li J, Qi W, Ampah KK, Ba X and Zeng X 2013 Actin polymerization negatively regulates p53 function by impairing its nuclear import in response to DNA damage. PLoS One 8 e60179
Xu J, Reumers J, Couceiro JR, De Smet F, Gallardo R, Rudyak S, Cornelis A, Rozenski J, et al. 2011 Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 7 285–295
Acknowledgements
This work was supported by the Natural Science Foundation of the Jilin Province Department of Science and Technology under Grant Number 20180520104JH; National Nature Science Foundation of China under Grant Number 31801182; and Natural Science Foundation of Changchun Normal University under Grant Numbers 2015-001 and 2016-001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Kundan Sengupta.
Corresponding editor: Kundan Sengupta
Rights and permissions
About this article
Cite this article
Qi, W., Li, J., Pei, X. et al. β-Actin facilitates etoposide-induced p53 nuclear import. J Biosci 45, 34 (2020). https://doi.org/10.1007/s12038-020-0004-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-020-0004-2